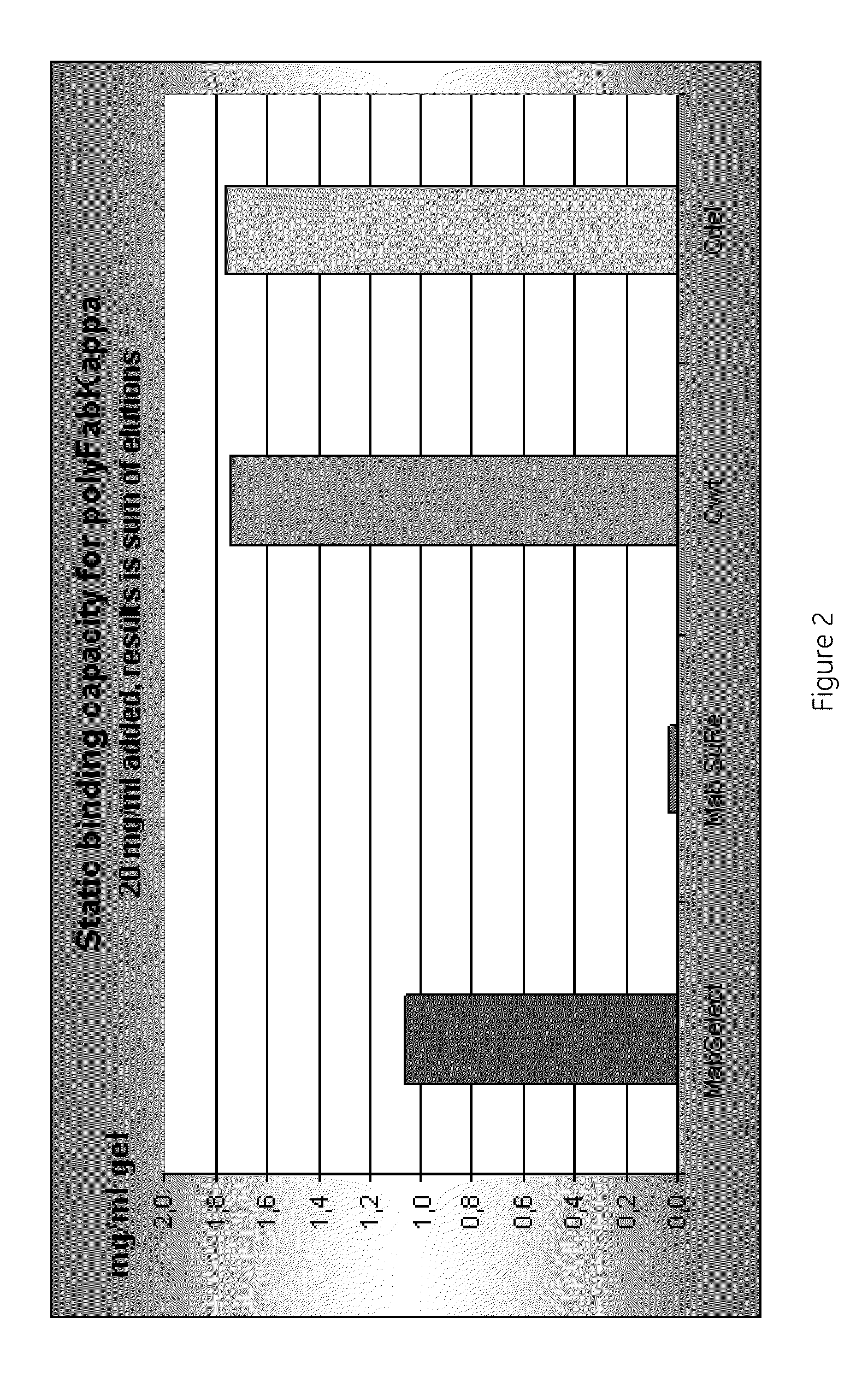
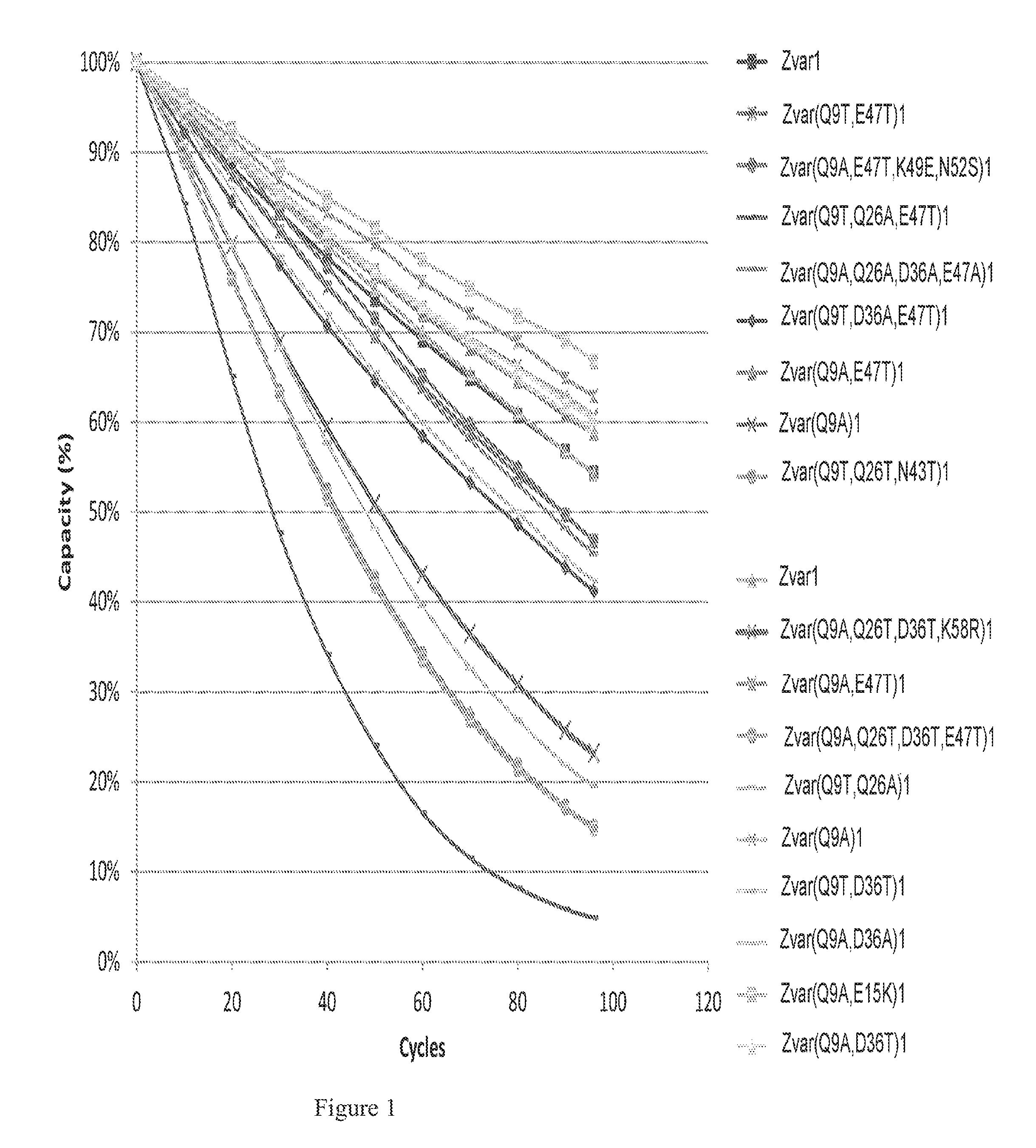
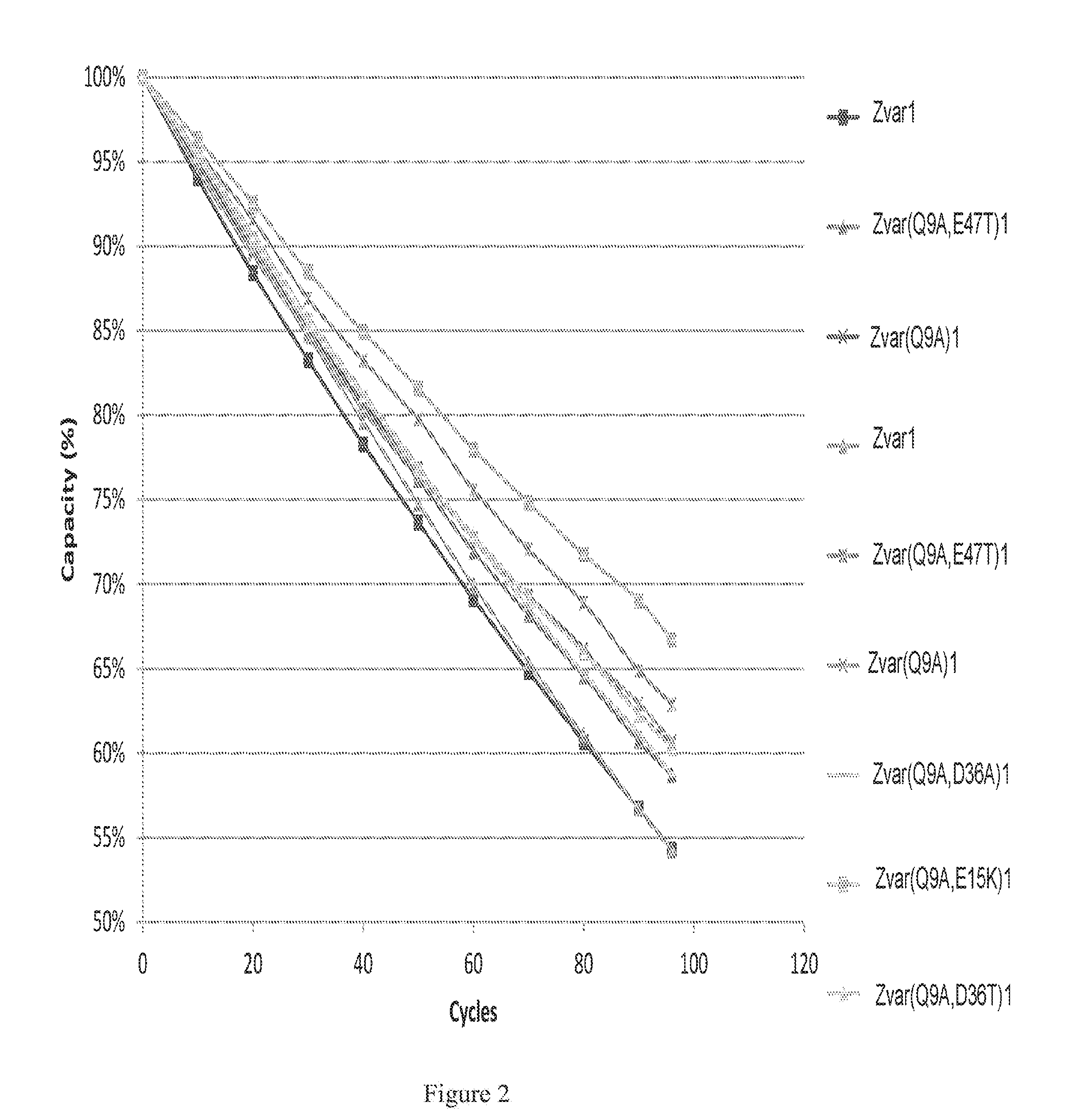
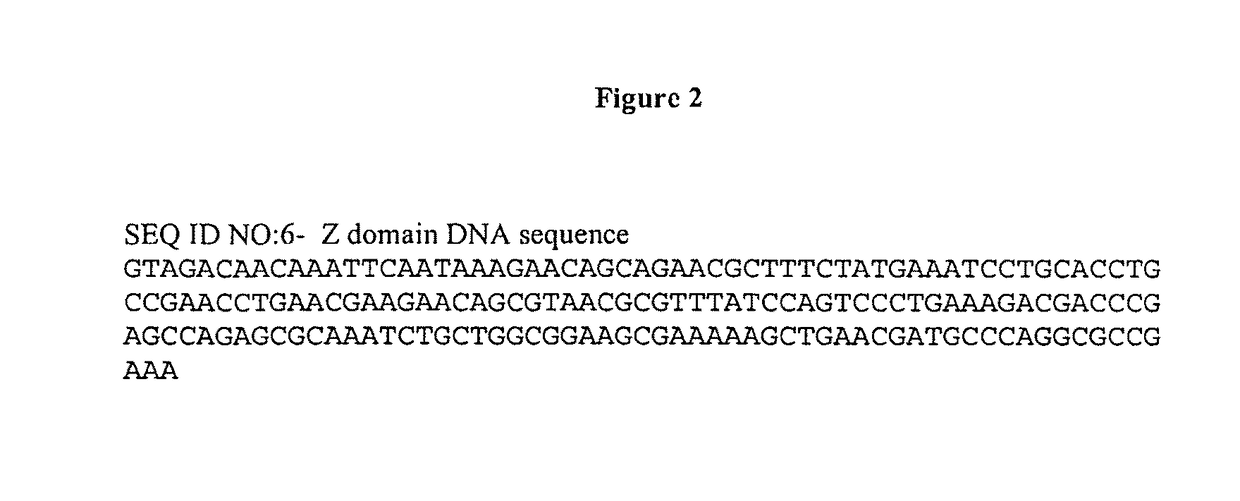
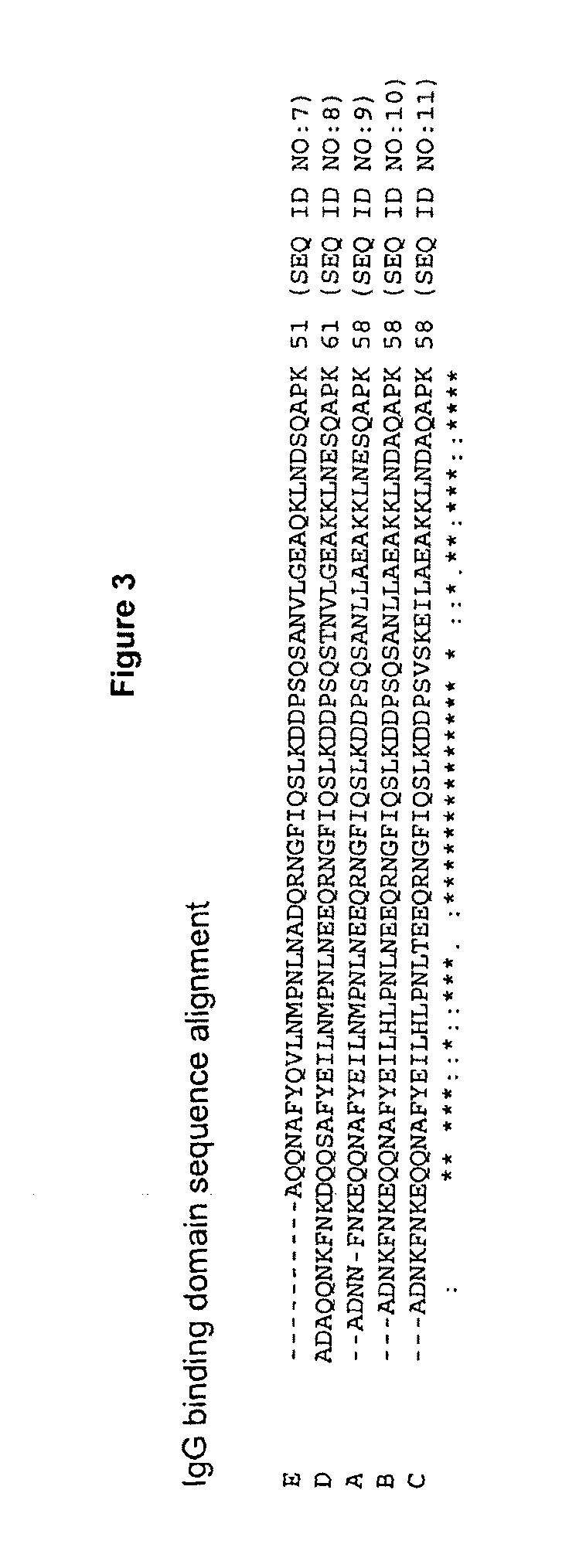
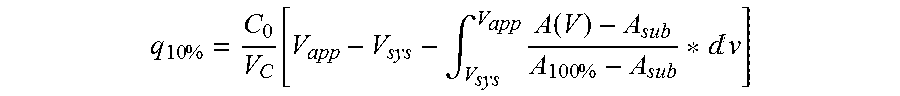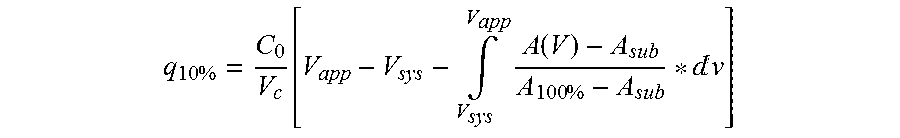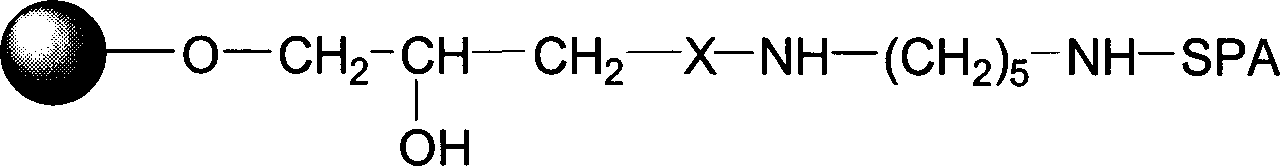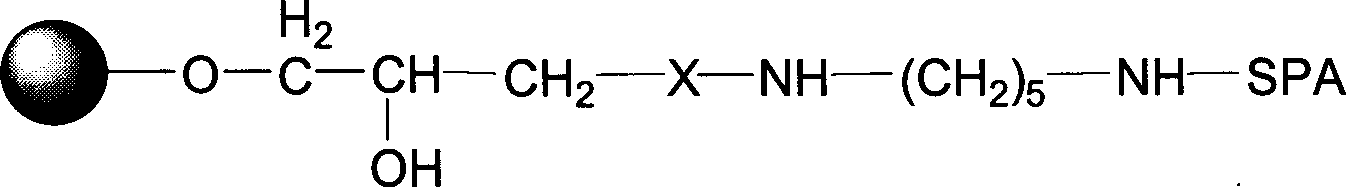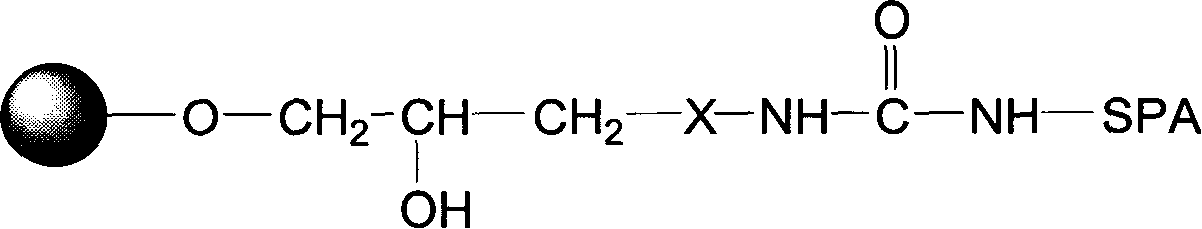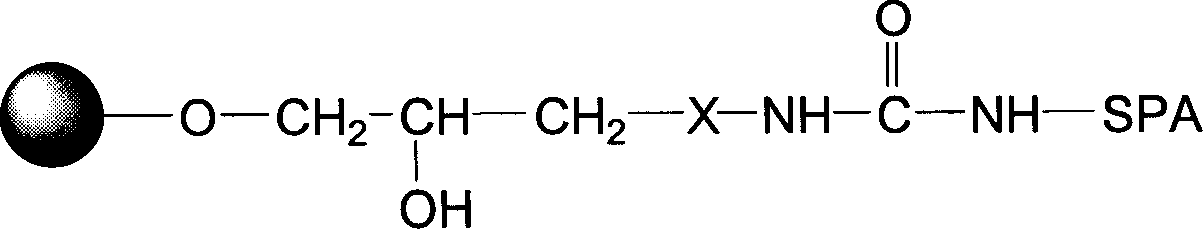Patents
Literature
52 results about "STAPH PROTEIN A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Staphylococcal protein A, Z Domain. Staphylococcal protein A, or SpA, is a type I membrane protein from the bacterium Staphylococcus aureus. It is bound to the cell wall via its C-terminal cell-wall-binding region X.
Chromatography ligand comprising domain C from Staphylococcus aureus protein A for antibody isolation
ActiveUS8329860B2Process economyPeptide/protein ingredientsSolid sorbent liquid separationArginineCoupling
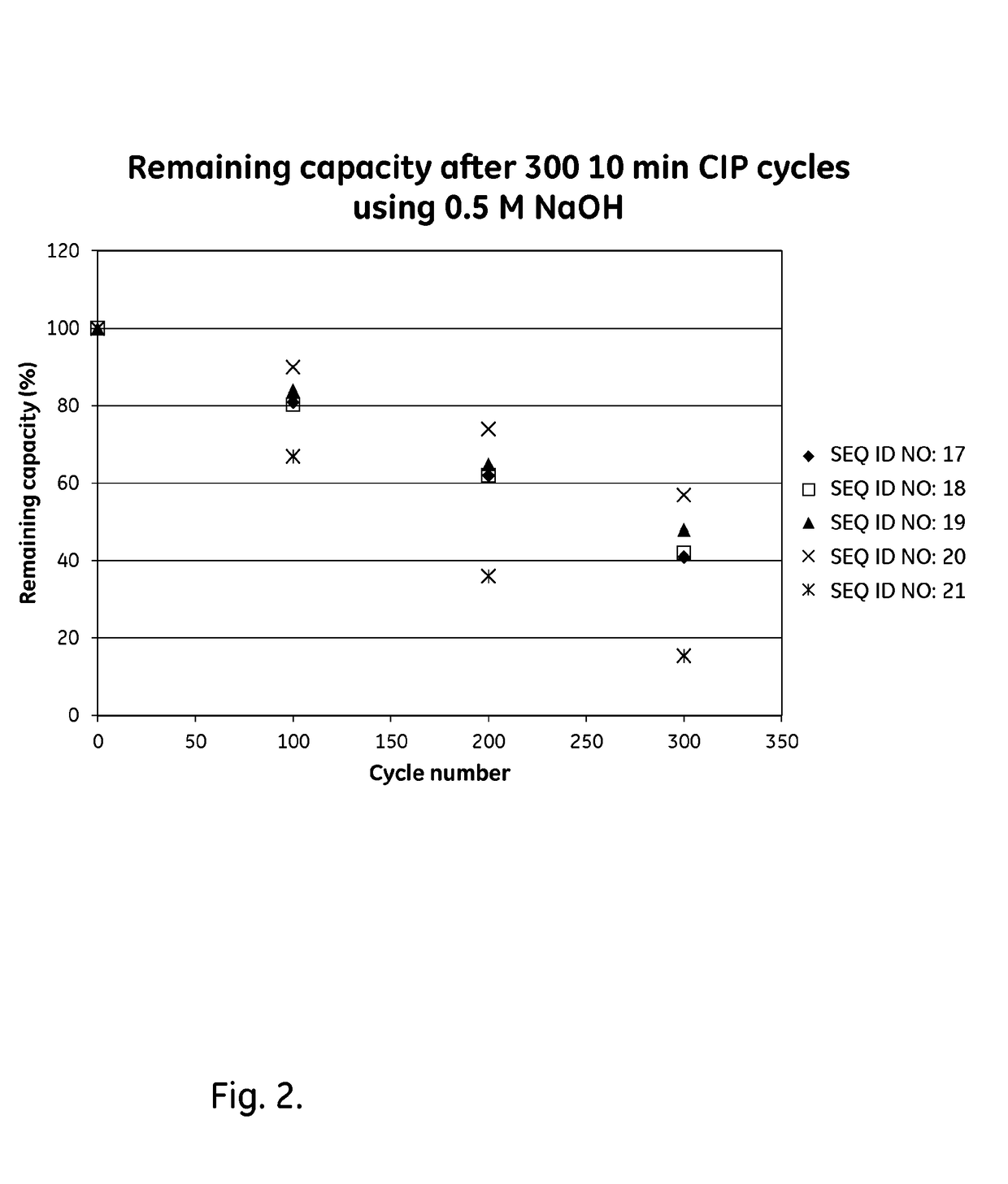
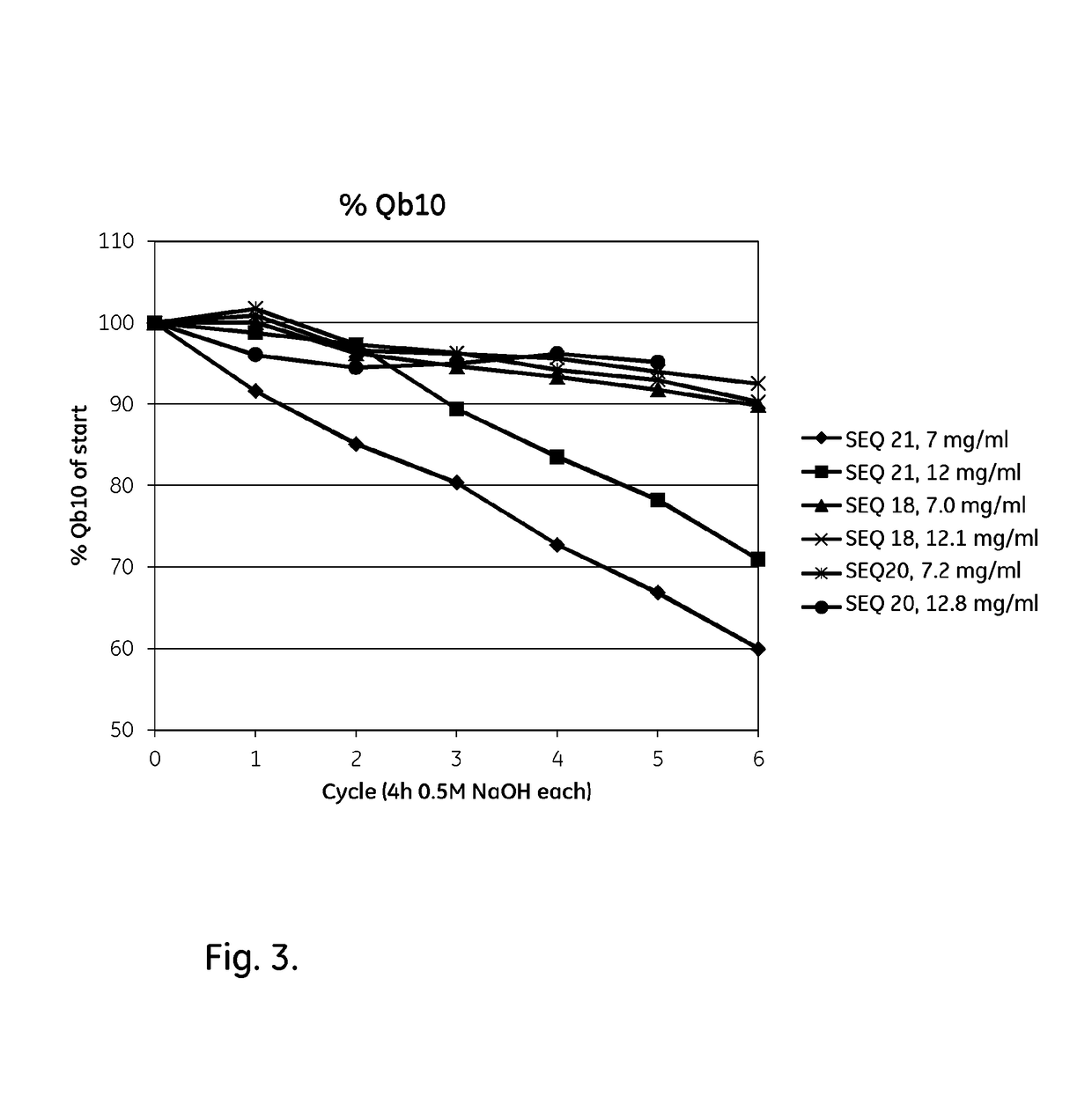
The present invention relates to a chromatography ligand, which comprises Domain C from Staphylococcus protein A (SpA), or a functional fragment or variant thereof. The chromatography ligand presents an advantageous capability of withstanding harsh cleaning in place (CIP) conditions, and is capable of binding Fab fragments of antibodies. The ligand may be provided with a terminal coupling group, such as arginine or cysteine, to facilitate its coupling to an insoluble carrier such as beads or a membrane. The invention also relates to a process of using the ligand in isolation of antibodies, and to a purification protocol which may include washing steps and / or regeneration with alkali.
Owner:CYTIVA BIOPROCESS R&D AB
Protein ligands
ActiveUS7709209B2Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionChemical stability
Owner:CYTIVA BIOPROCESS R&D AB
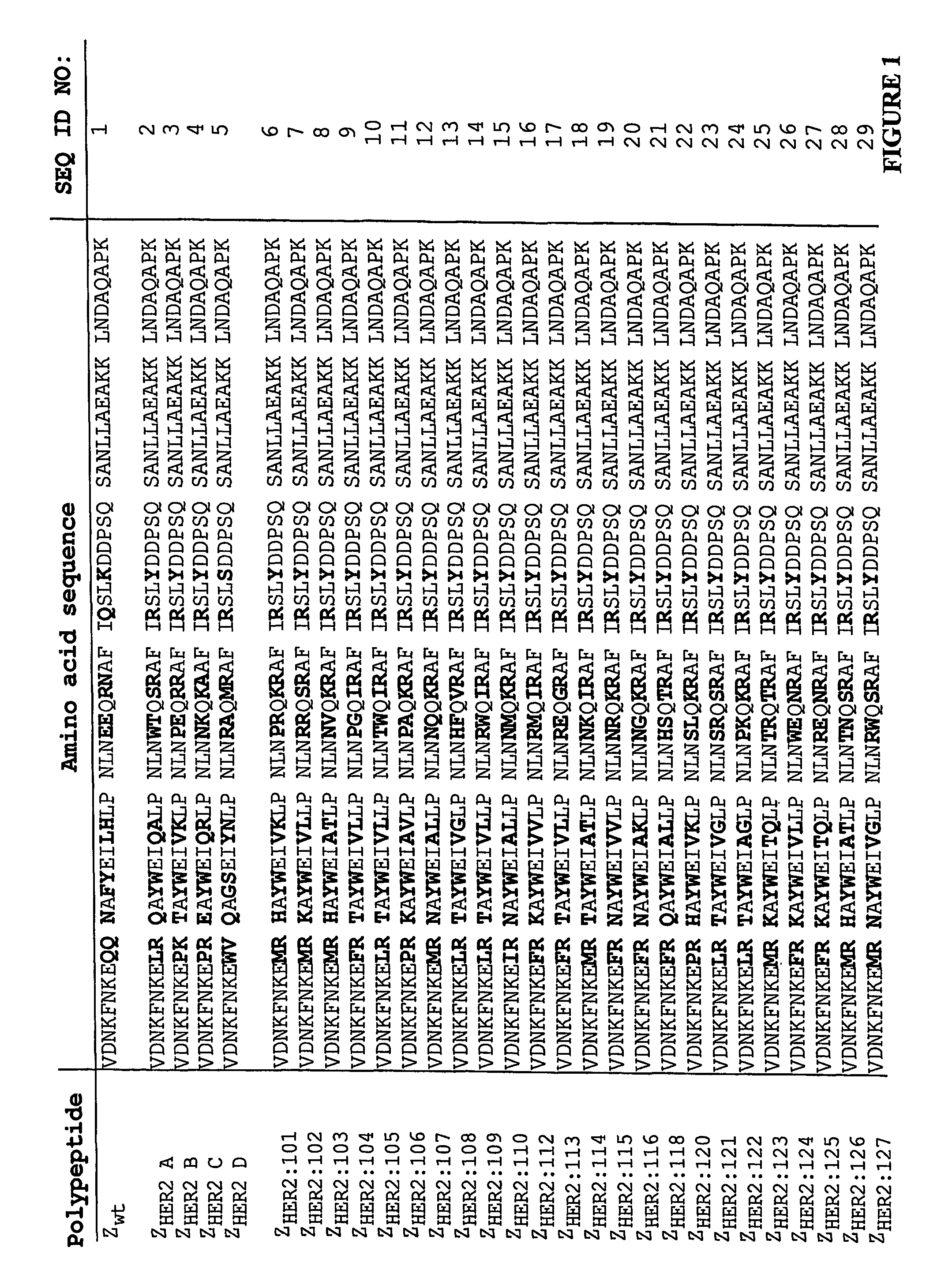
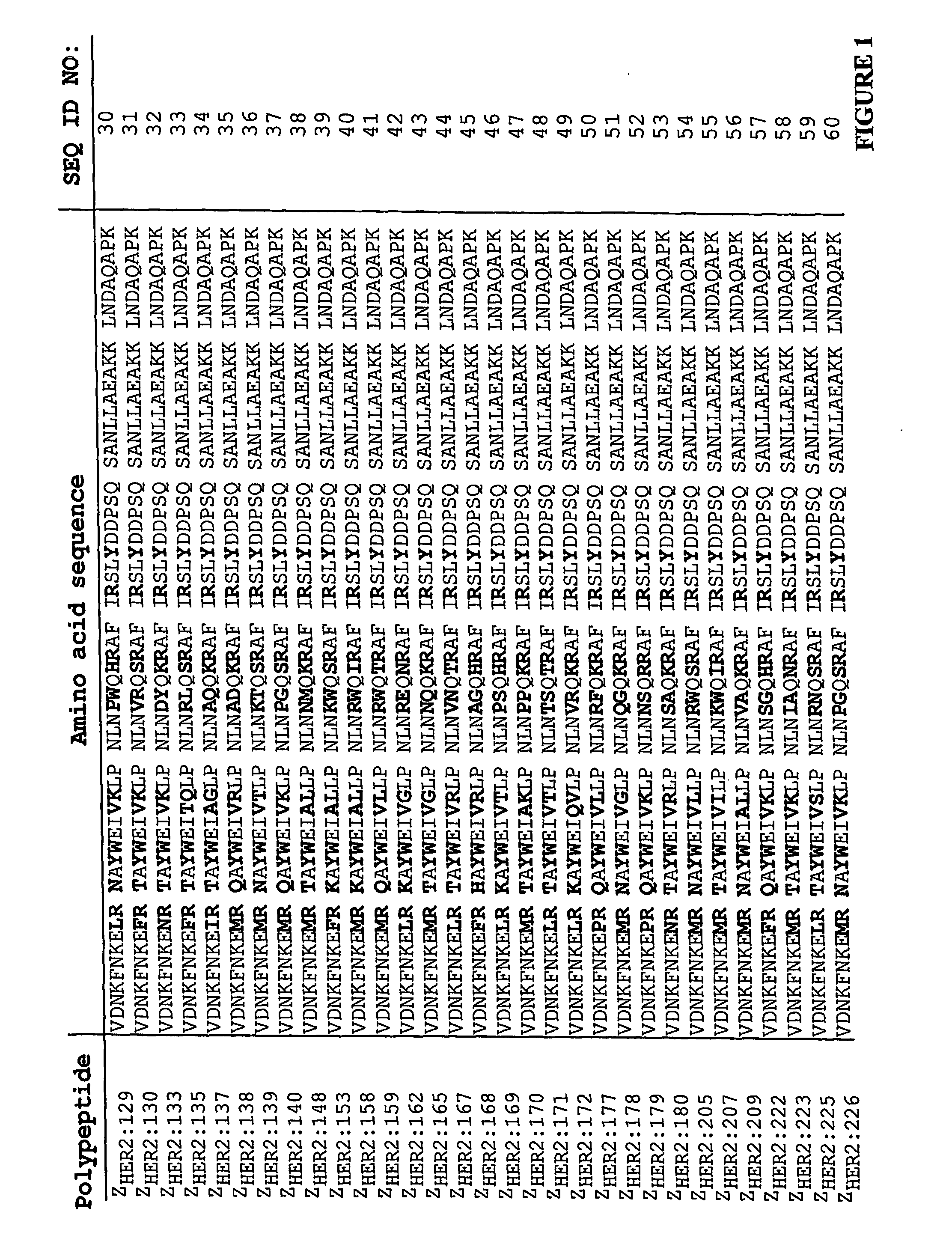
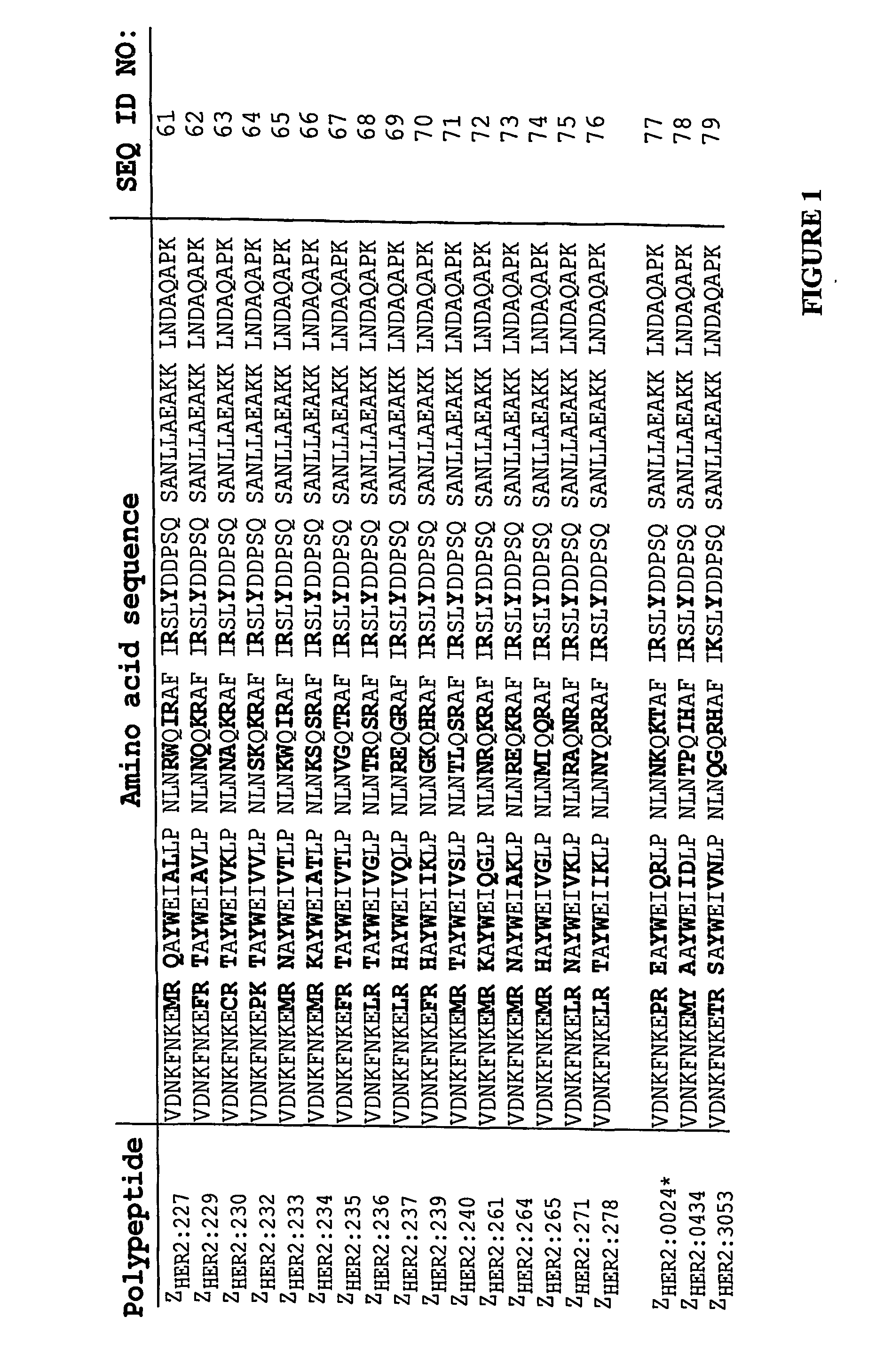
Polypeptides having binding affinity for HER2
ActiveUS7993650B2Easy to useImprove bindingPeptide/protein ingredientsImmunoglobulinsDirect substanceA domain
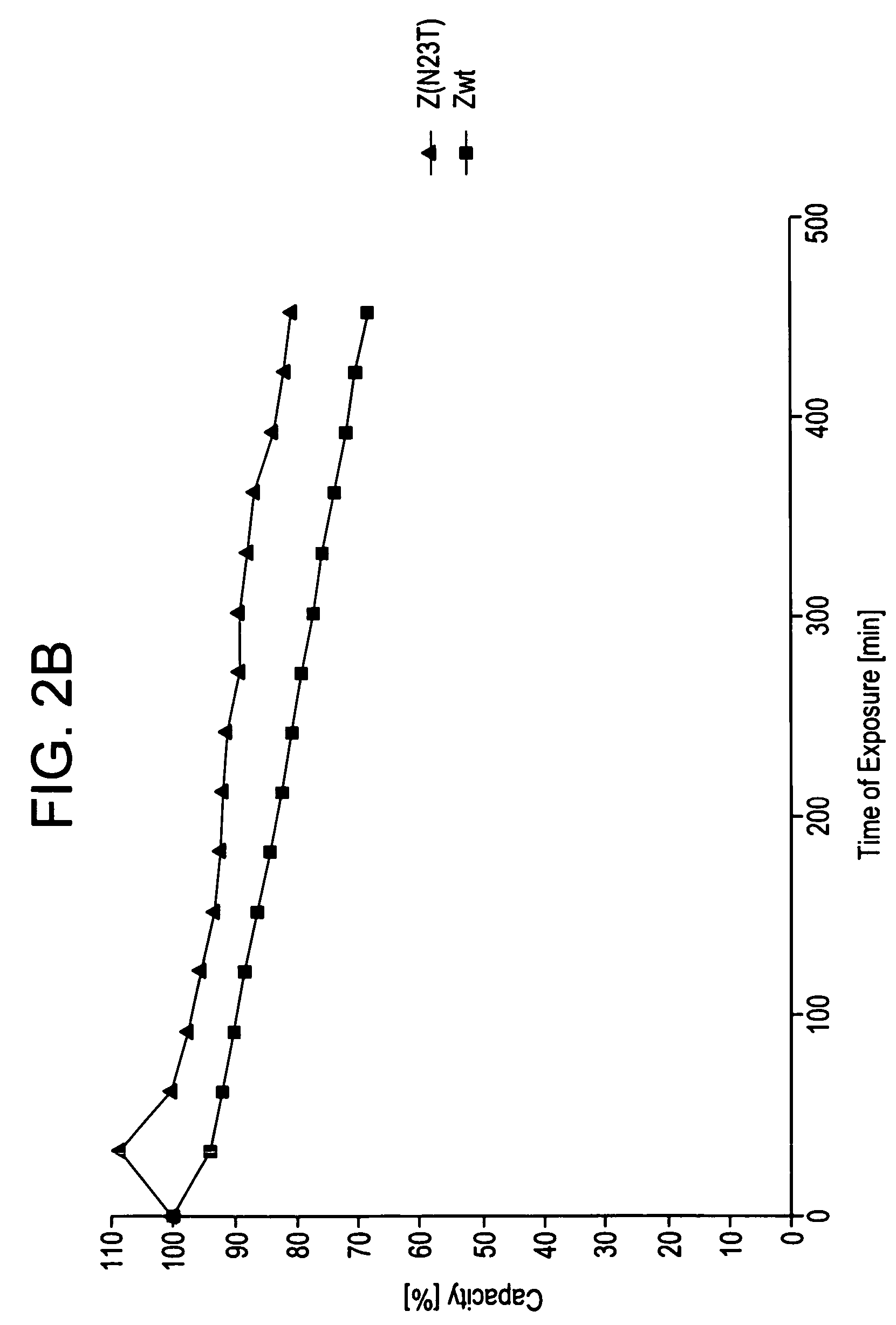
A polypeptide is provided, which has a binding affinity for HER2 and which is related to a domain of staphylococcal protein A (SPA) in that the sequence of the polypeptide corresponds to the sequence of the SPA domain having from 1 to about 20 substitution mutations. Nucleic acid encoding the polypeptide, as well as expression vector and host cell for expressing the nucleic acid, are also provided. Also provided is the use of such a polypeptide as a medicament, and as a targeting agent for directing substances conjugated thereto to cells overexpressing HER2. Methods, and kits for performing the methods, are also provided, which methods and kits rely on the binding of the polypeptide to HER2.
Owner:AFFIBODY TECH AB
Mutated Immunoglobulin-Binding Polypeptides
ActiveUS20170334954A1Improved alkaline stabilityImprove stabilityHybrid immunoglobulinsSolid sorbent liquid separationArginineFc binding
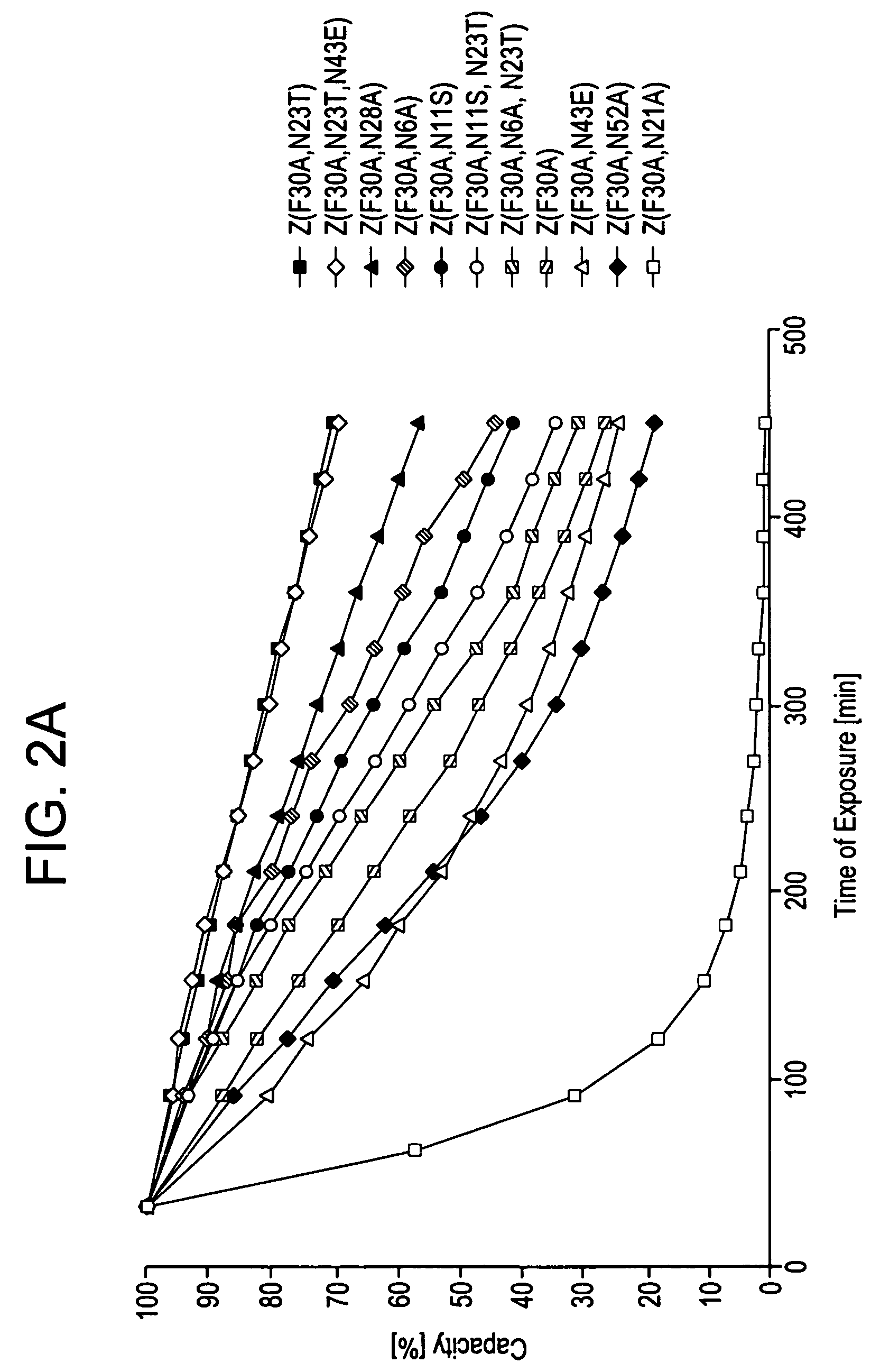
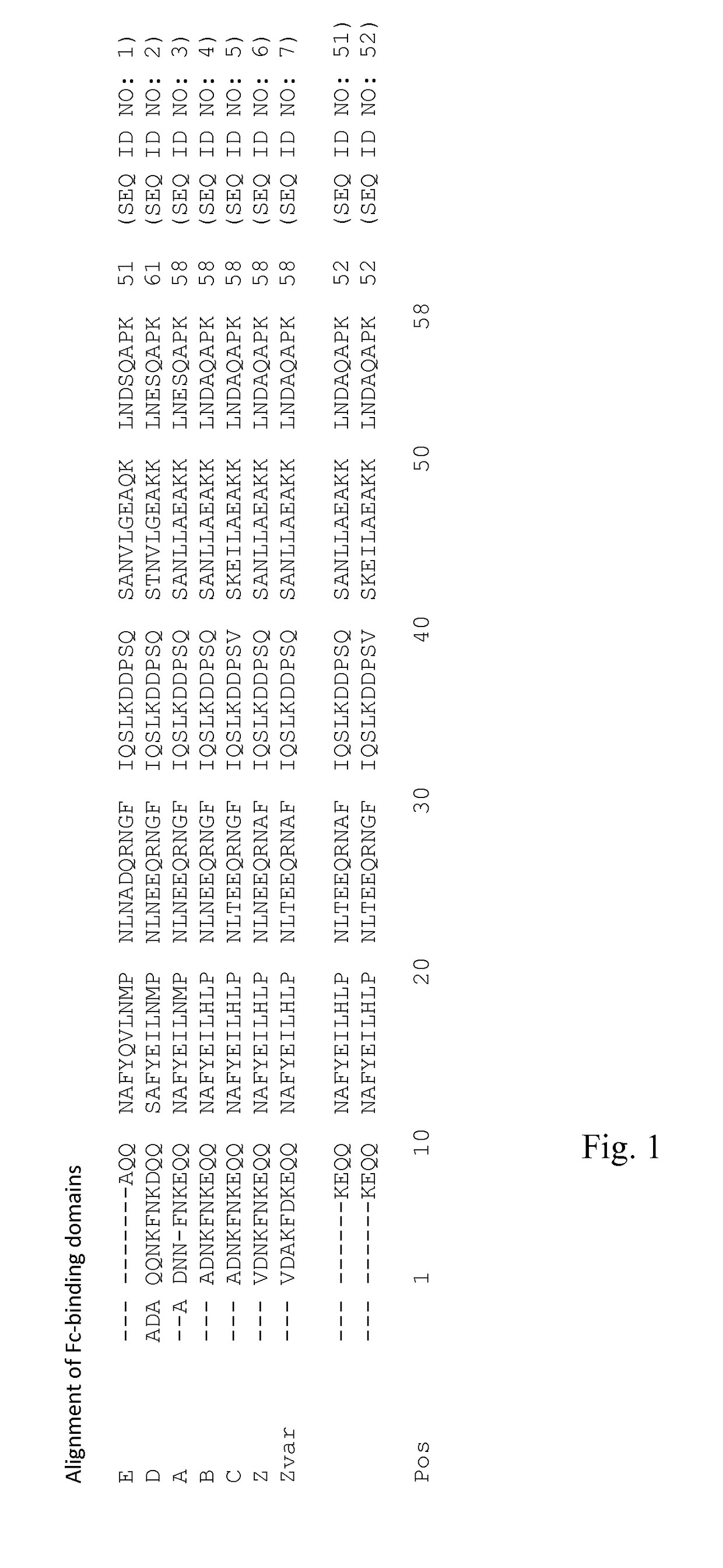
An Fc-binding polypeptide of improved alkali stability, comprising a mutant of an Fc-binding domain of Staphylococcus Protein A (SpA), as defined by SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO:3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:22, SEQ ID NO 51 or SEQ ID NO 52 wherein at least the asparagine or serine residue at the position corresponding to position 11 in SEQ ID NO:4-7 has been mutated to an amino acid selected from the group consisting of glutamic acid, lysine, tyrosine, threonine, phenylalanine, leucine, isoleucine, tryptophan, methionine, valine, alanine, histidine and arginine.
Owner:CYTIVA BIOPROCESS R&D AB
Chromatography ligand comprising domain c from staphylococcus aureus protein a for antibody isolation
ActiveUS20100048876A1Process economyPeptide/protein ingredientsOther chemical processesArginineCoupling
The present invention relates to a chromatography ligand, which comprises Domain C from Staphylococcus protein A (SpA), or a functional fragment or variant thereof. The chromatography ligand presents an advantageous capability of withstanding harsh cleaning in place (CIP) conditions, and is capable of binding Fab fragments of antibodies. The ligand may be provided with a terminal coupling group, such as arginine or cysteine, to facilitate its coupling to an insoluble carrier such as beads or a membrane. The invention also relates to a process of using the ligand in isolation of antibodies, and to a purification protocol which may include washing steps and / or regeneration with alkali.
Owner:CYTIVA BIOPROCESS R&D AB
Mutated Immunoglobulin-Binding Polypeptides
ActiveUS20160159857A1Highly selective bindingImproved alkaline stabilitySolid sorbent liquid separationDepsipeptidesTryptophanMutant
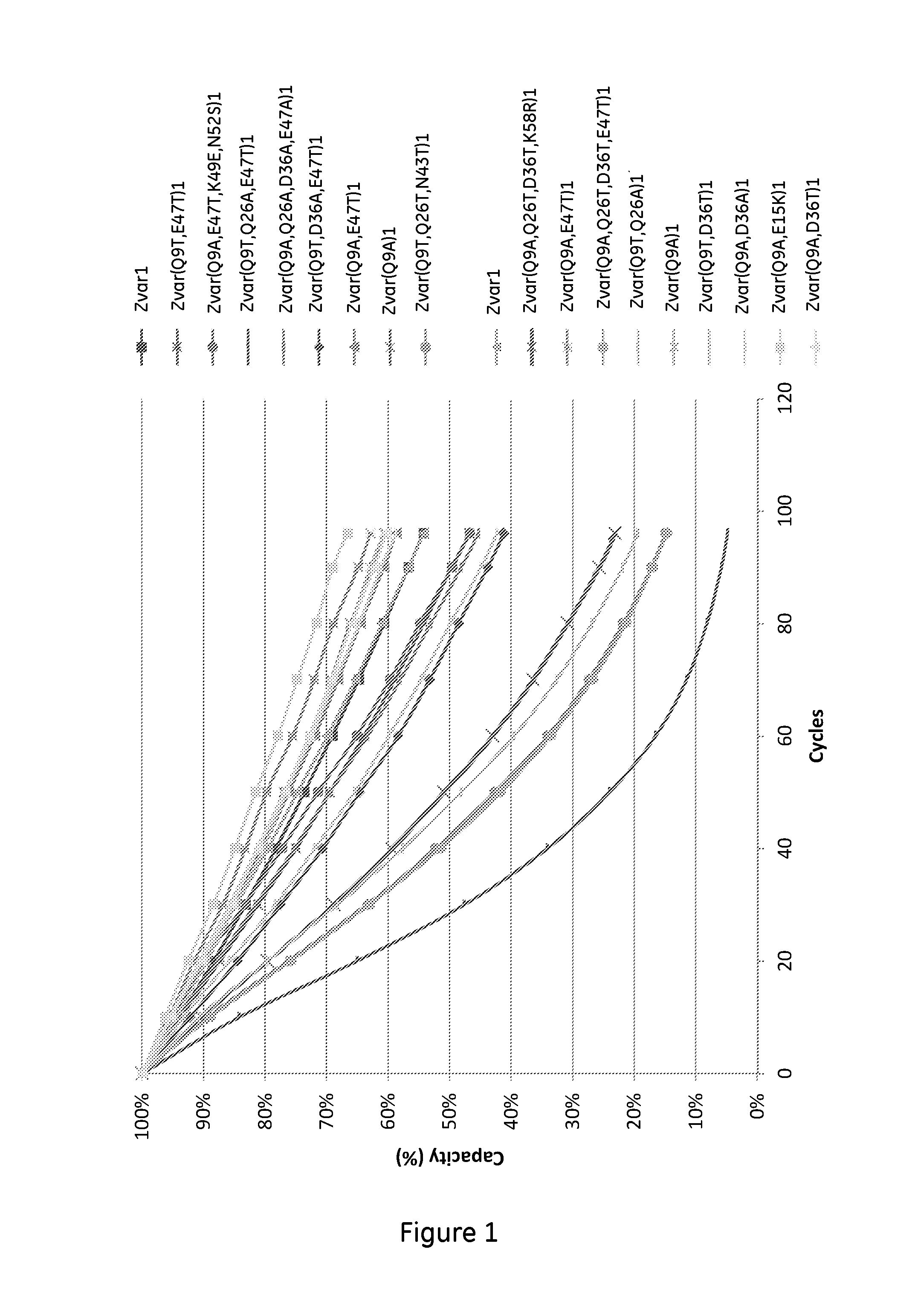
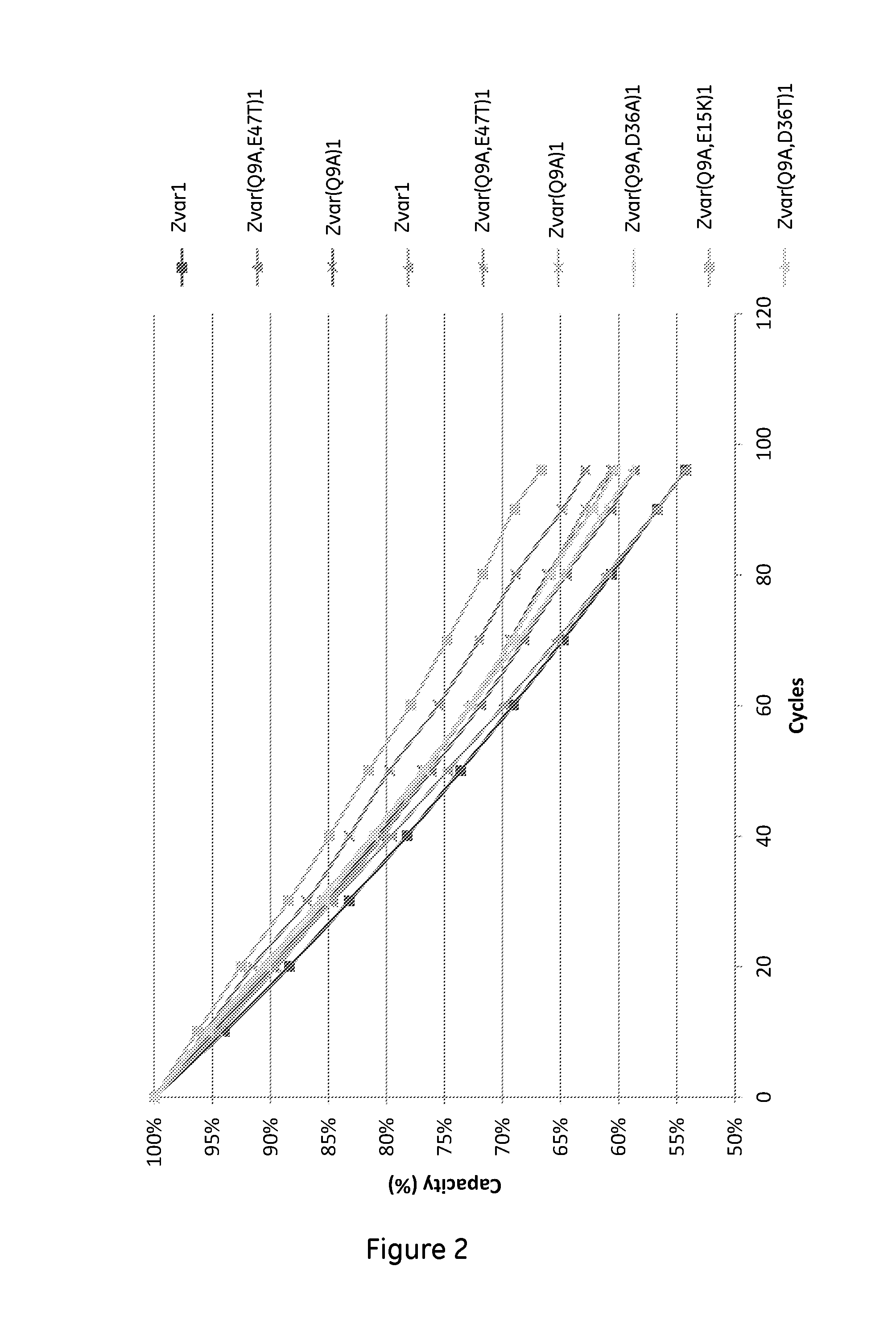
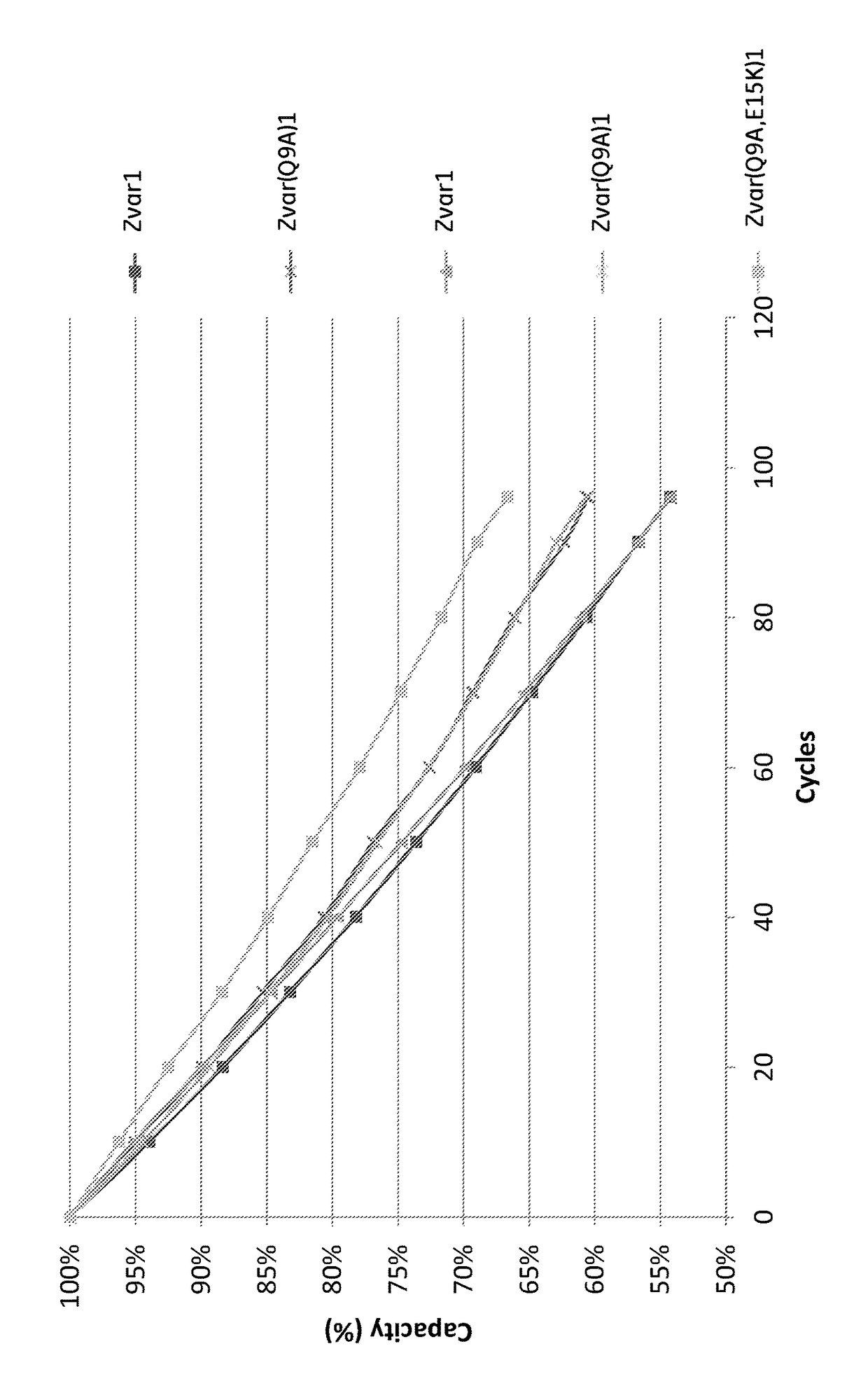
The invention discloses a polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A (SpA), as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, comprising at least the mutation wherein the glutamine residue at position 9 has been mutated to a tryptophan, leucine, glutamic acid, valine or lysine. The invention also discloses multimers of the polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
Mutated immunoglobulin-binding polypeptides
ActiveUS20160159855A1Improved alkaline stabilityHighly selectiveSugar derivativesPeptide/protein ingredientsImmunoglobulin IgEMutant
A polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A, as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, wherein at least the glutamine residue at position 9 has been mutated to an amino acid other than asparagine. The invention also discloses multimers of said polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
Protein A based binding domains with desirable activities
InactiveUS7163686B1Reduce in quantityGood treatment effectBiocideBacterial antigen ingredientsHeavy chainBinding domain
Provided are Staphylococcal protein A (SpA) variants for binding immunoglobulin (Ig), comprising a polypeptide which varies by one or more amino acids from the amino acid sequence of a natural variable heavy chain III (“VH3”) Ig-Fab binding region (“binding region”) of SpA, wherein the polypeptide exhibits a different binding specificity for Ig-Fab than does SpA or exhibits a different binding specificity for a non-Ig target molecule than does SpA. Further provided are methods of making the variants and methods of using the variants for in purification of Ig as well as diagnostic and therapeutic intervention.
Owner:RGT UNIV OF CALIFORNIA
Novel immobilizing fusion protein for effective and oriented immobilization of antibody on surfaces
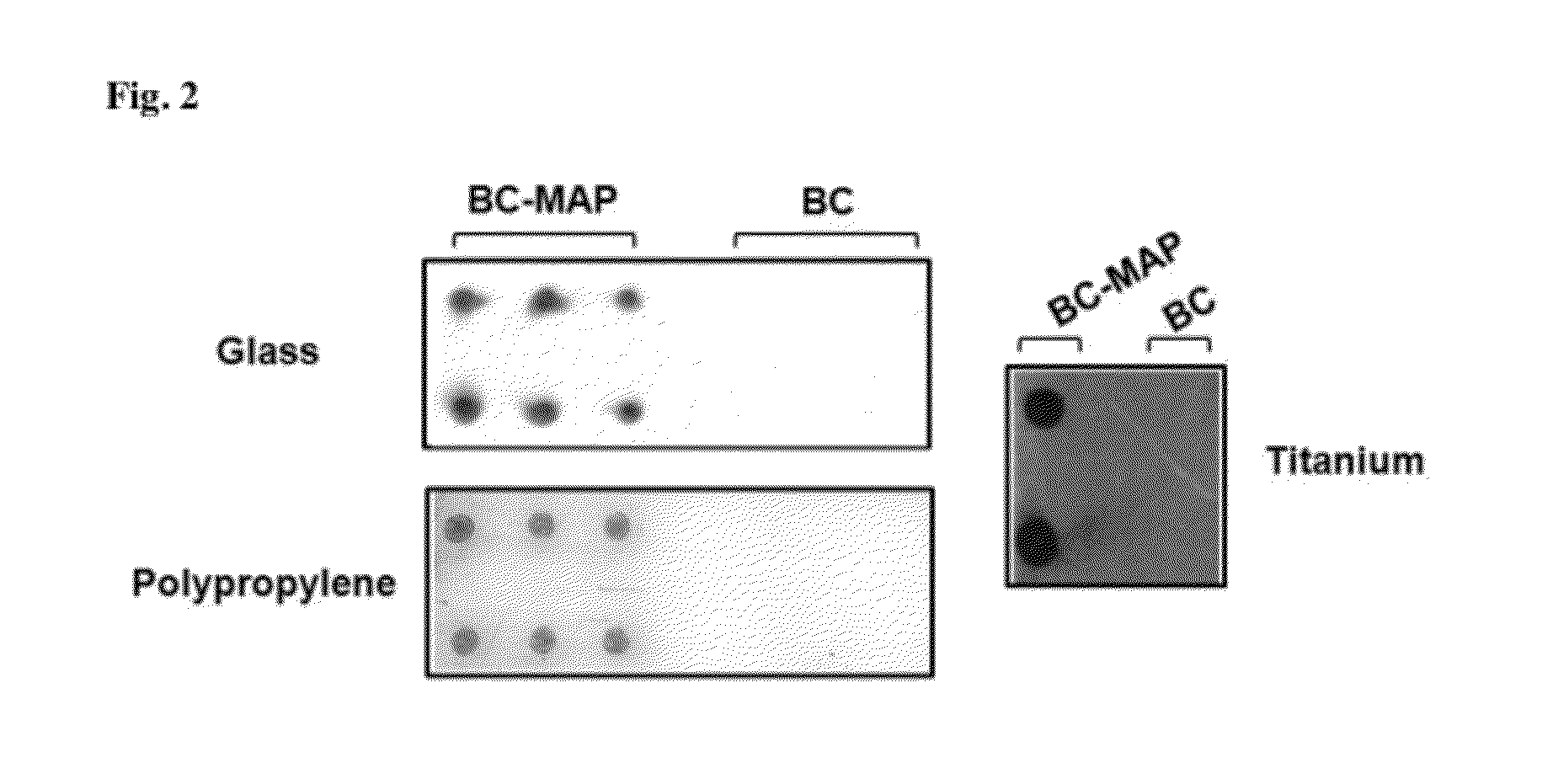
The present invention relates to a novel fusion protein comprising Staphylococcal protein A and mussel adhesive protein, a biochip comprising a solid substrate to which the fusion protein is attached, and a method for detecting a target antigen in a biological sample using the biochip. Furthermore, the present invention relates to a polynucleotide encoding the fusion protein, a recombinant vector comprising the polynucleotide, a transformed cell comprising the recombinant vector, and a method of preparing the fusion protein by transformed cell comprising the recombinant vector.
Owner:POSTECH ACAD IND FOUND
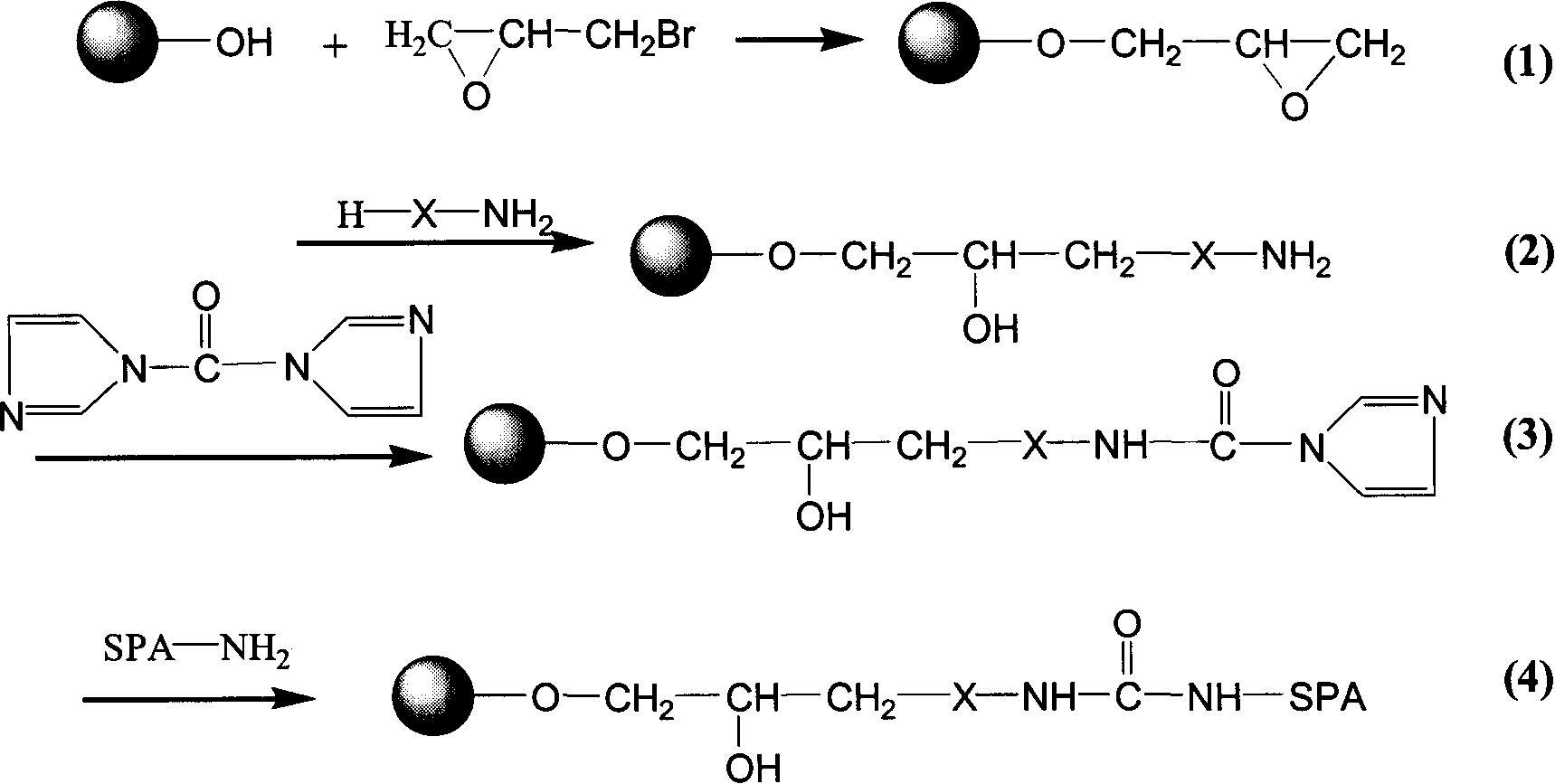
Protein A immunoadsorption material for eliminating pathogenic antibody and its complexes, and synthesizing method and application thereof
ActiveCN101185878AImprove adsorption capacityHigh chemical activityOther chemical processesEpoxyDouble bond
The invention provides a protein A immunoadsorption material which eliminates pathogenic antibody and the compound from plasma and the a preparation method. The invention discloses a polymer material coupled by agarose gel and staphylococcal protein A (SPA) and the material takes the agarose gel as vector matrix and takes polyamines reagent as a space arm, then reacting with glutaraldehyde and finally coupling with the protein A and blocking, reducing a double bond after being activated by epoxy bromopropane. The product has the high safety; by making use of the produce, the pathogenic antibody, more particularly, the immune compound pathogenic antibody can be effectively and selectively eliminated from the plasma of patients; and the invention can be applied to clinical immunoadsorption treatments.
Owner:GUANGZHOU KONCEN BIOSCI +1
Polypeptides Having Binding Affinity for Her2
ActiveUS20100048868A1Improve stabilityReduce sensitivityBacteriaPeptide/protein ingredientsDirect substanceA domain
A polypeptide is provided, which has a binding affinity for HER2 and which is related to a domain of staphylococcal protein A (SPA) in that the sequence of the polypeptide corresponds to the sequence of the SPA domain having from 1 to about 20 substitution mutations. Nucleic acid encoding the polypeptide, as well as expression vector and host cell for expressing the nucleic acid, are also provided. Also provided is the use of such a polypeptide as a medicament, and as a targeting agent for directing substances conjugated thereto to cells overexpressing HER2. Methods, and kits for performing the methods, are also provided, which methods and kits rely on the binding of the polypeptide to HER2.
Owner:AFFIBODY TECH AB
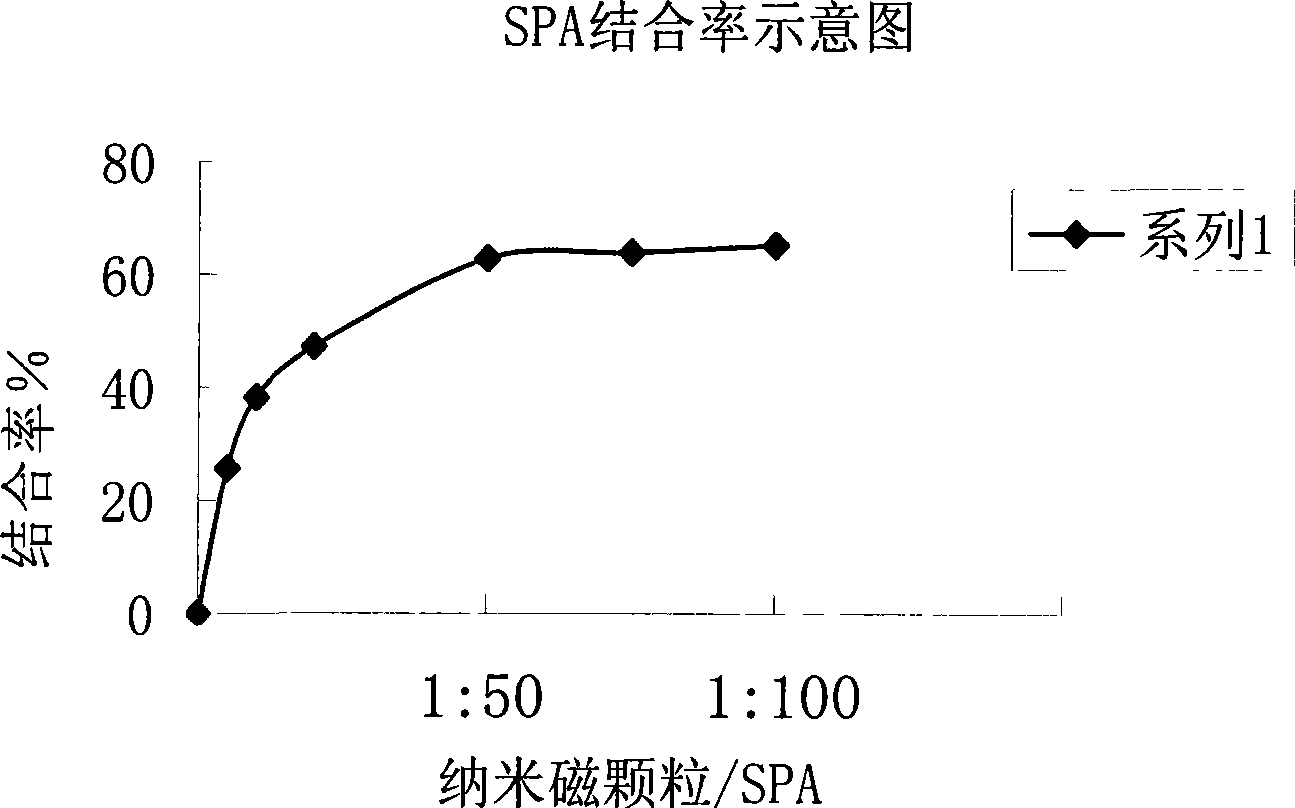
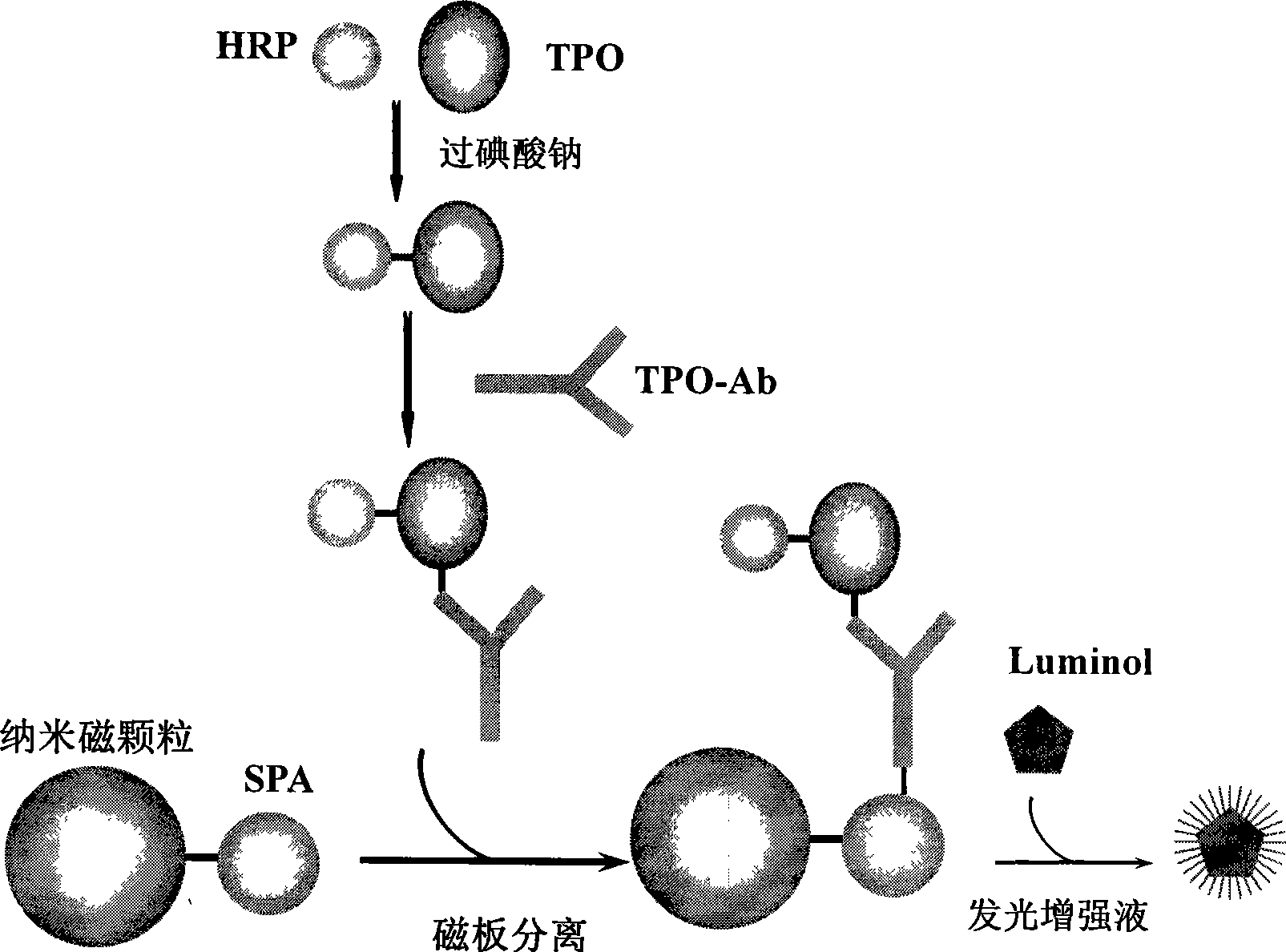
Chemiluminescent ligand analysis method for quantitative detection of human auto-antibody
InactiveCN101470117ASolve the problem of inaccurate quantitative detectionNo cross-reactivityBiological testingAutoantibodyBiomedical technology
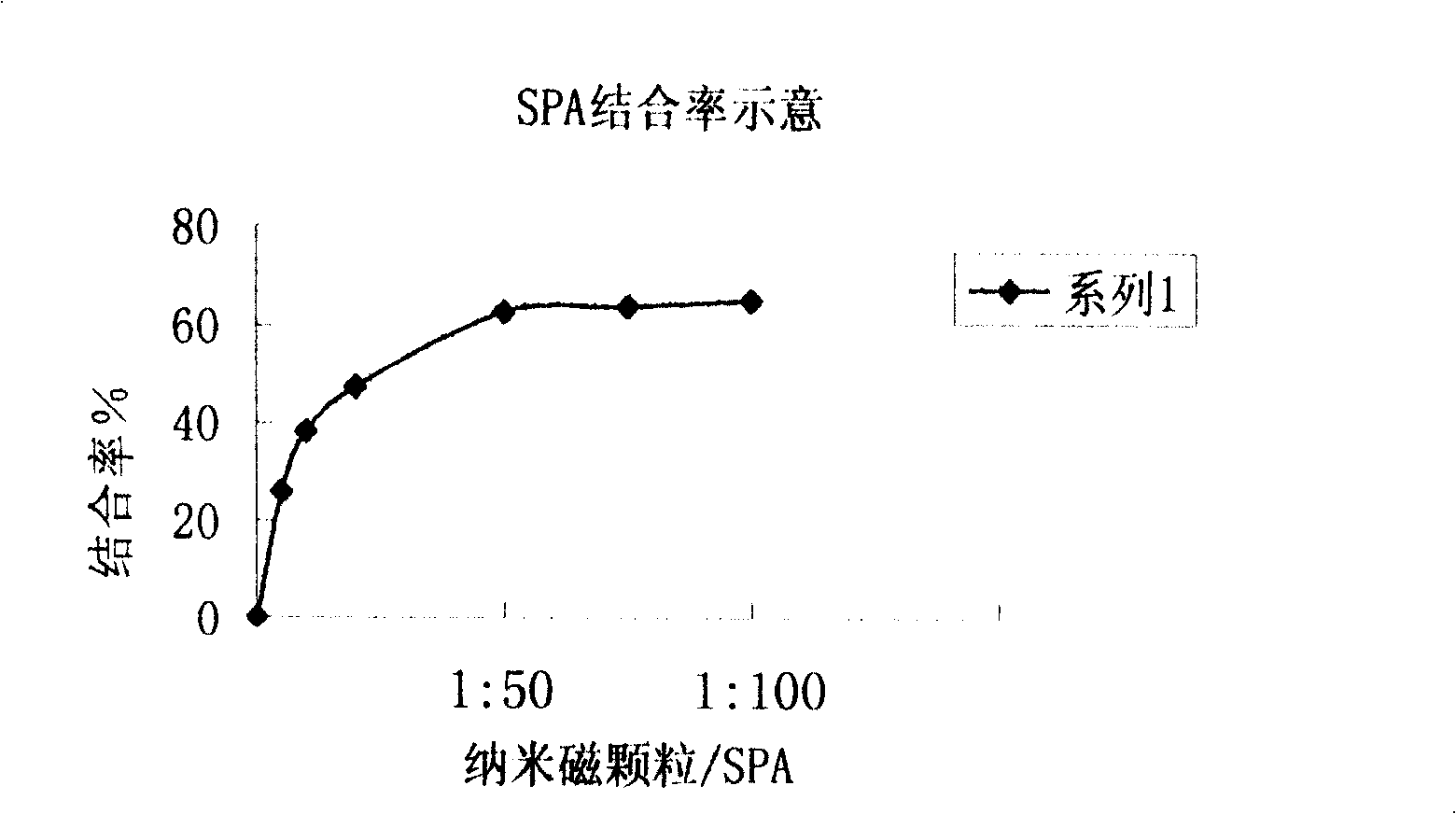
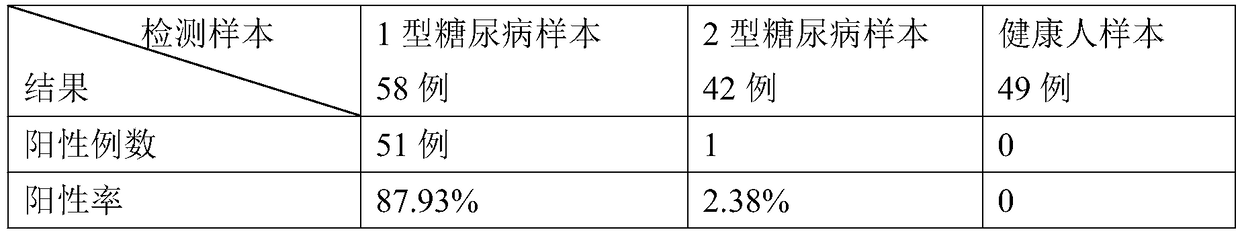
A chemical luminous ligand analysis method for quantitatively checking human antibodies belongs to the biomedical technical field, which comprises: using the generality that staphylococcal protein A (SPA) can react with Fc point of IgG in mice; using the monoclonal antibody of high affinity and specificity to prepare a standard product to establish a standard curve; respectively reacting the monoclonal antibody and the antibody in the sample with enzyme-labeled antigens; using the nanometer magnetic particles coated by SPA as ligand to separate solid and liquid; and using enzymatic chemical illumination reaction catalyzed by horseradish peroxidase (HRP) to process illumination check; thereby establishing a chemical luminous ligand quantitative analysis on human antibodies. The chemical luminous ligand analysis method is suitable for quantitatively checking all human antibodies, for resolving the problem of prior art while most human antibodies can not be accurately and quantitatively checked, avoiding cross reaction and avoiding false negative result or false positive result.
Owner:天津市协和医药科技集团有限公司
Hydrogel material for repairing central nervous and preparation thereof
InactiveCN101301494AGood biocompatibilityWith mechanical propertiesSurgeryPolyethylene glycolBiocompatibility Testing
The invention discloses a hydrogel material for nervus centralis repair and a method for making the same. The method comprises the following steps of: mixing polyethylene glycol water solution with density of 1 to 5 weight percent with polylysine or staphylococcal protein A and nanometer ferric oxide water solution with the density of 0.1 to 2 weight percent, oscillating and stirring; adding glutaral pentanedial solution until reaching final density of 1 to 4 weight percent of the glutaral pentanedial, and reacting for 2 to 24 hours below 4 DEG C; freezing and drying; and cleaning as well as renewly freezing and drying. The hydrogel material for nervus centralis repair made in the method has the advantages of good biocompatibility, rheological characteristics and mechanical properties similar to the nervus centralis tissue, no secondary damage to tissue after implantation of the nervus centralis, and is capable of regulating biodegradability in a large scale and is applicable to nervus repair. And the making method has simple process, low cost, and is suitable for large-scale production.
Owner:CENT SOUTH UNIV
Blood purifying protein A immunoadsorption material and synthesizing method thereof
ActiveCN101190409AHigh chemical activityImprove adsorption capacityOther chemical processesEpoxySynthesis methods
The invention relates to a staphylococcal protein A (SPA) immunoadsorption material used for blood purification and a preparation method thereof. The invention discloses a macromolecule material which is coupled by agarose gel and SPA. The material is prepared by taking the agarose gel as carrier substrate which is reacted with epoxy bromopropane to obtain the epoxy-based active carrier; after that, polyamines reagent is taken as a space arm which is then reacted with carbonyl diimidazole and is then coupled with the SPA. The material has the advantages of short synthesis time, safe preparation, strong specificity of product, high adsorption efficiency and good regeneration performance to immunoglobulin and the compound thereof, and being able to be applied to clinic immunoadsorption cure.
Owner:GUANGZHOU KONCEN BIOSCI +1
Affinity chromatography matrix
ActiveUS20150080554A1Optimize purification stepsInhibition formationOther chemical processesSolid sorbent liquid separationThreonineHistidine
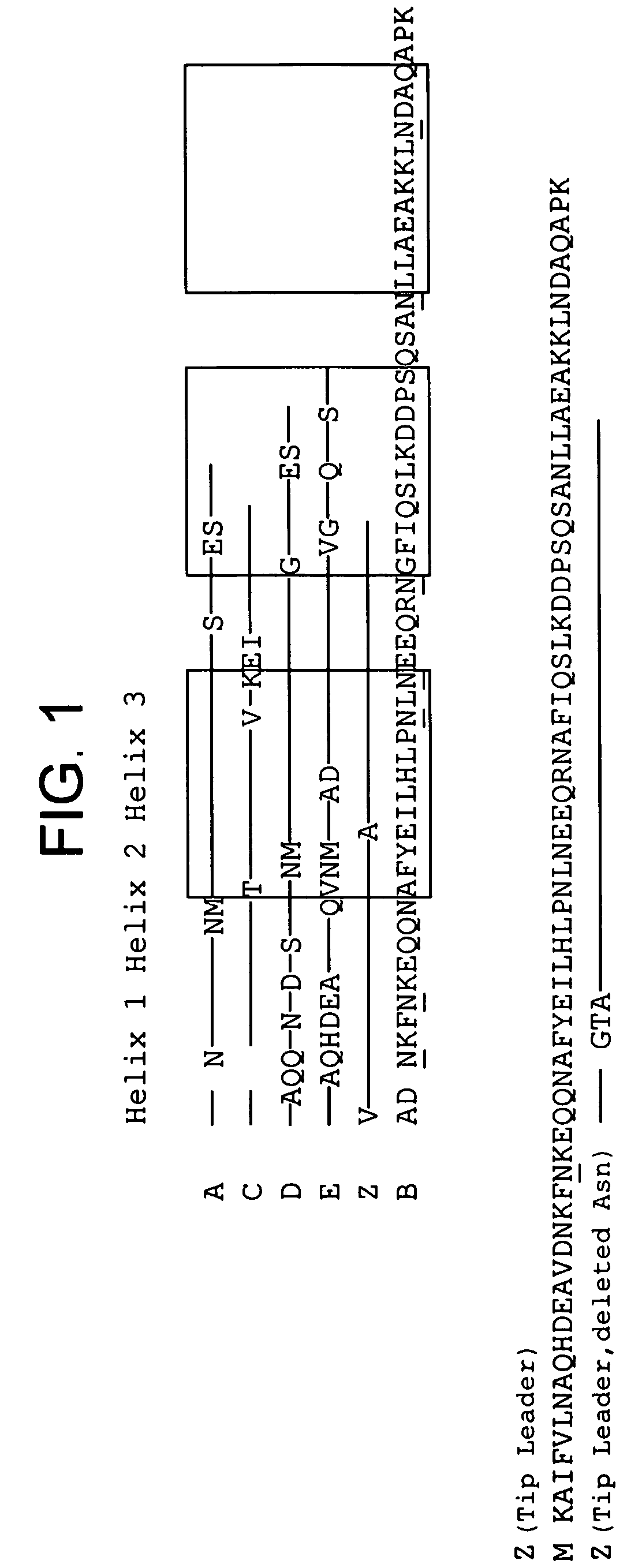
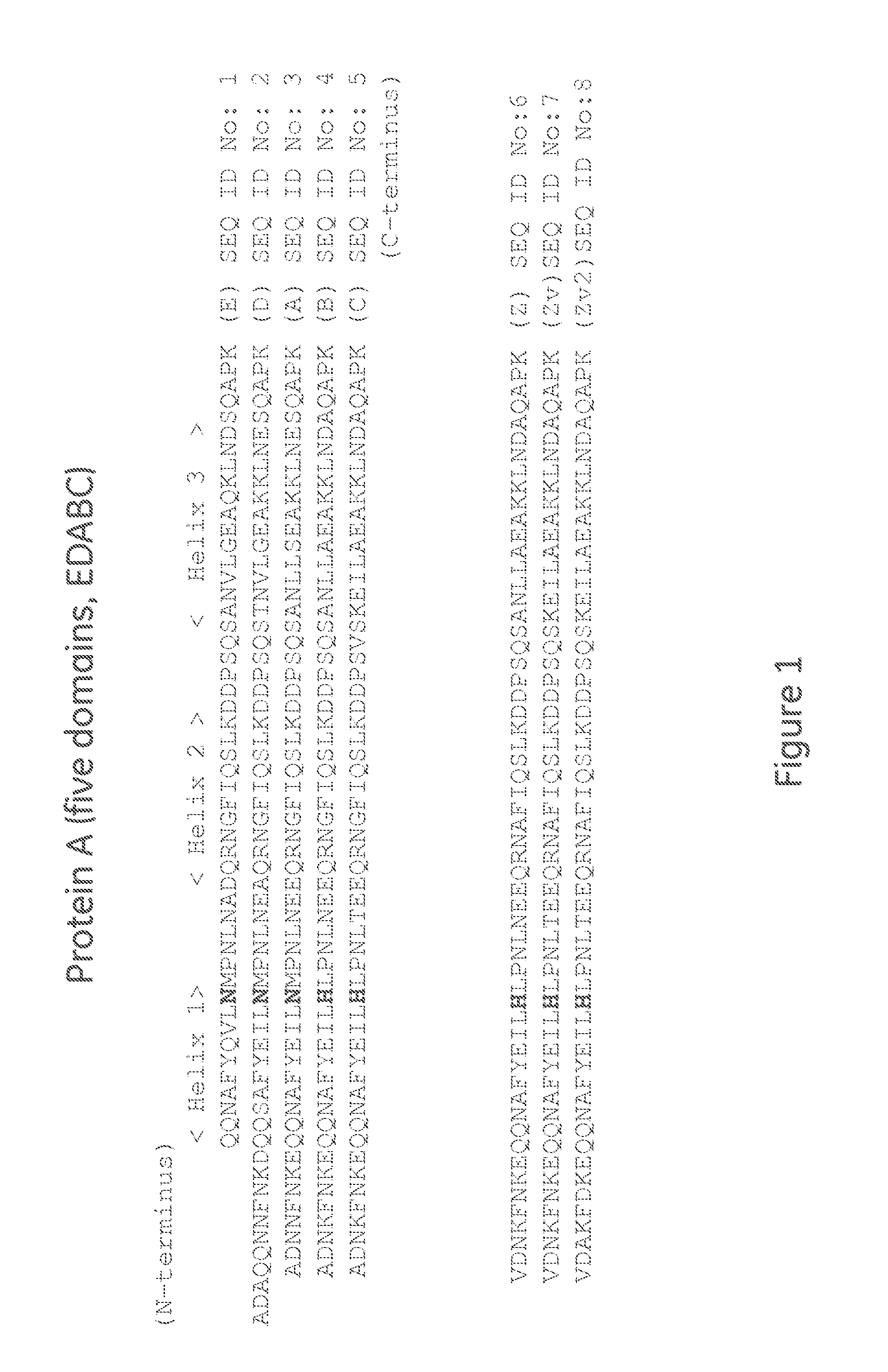
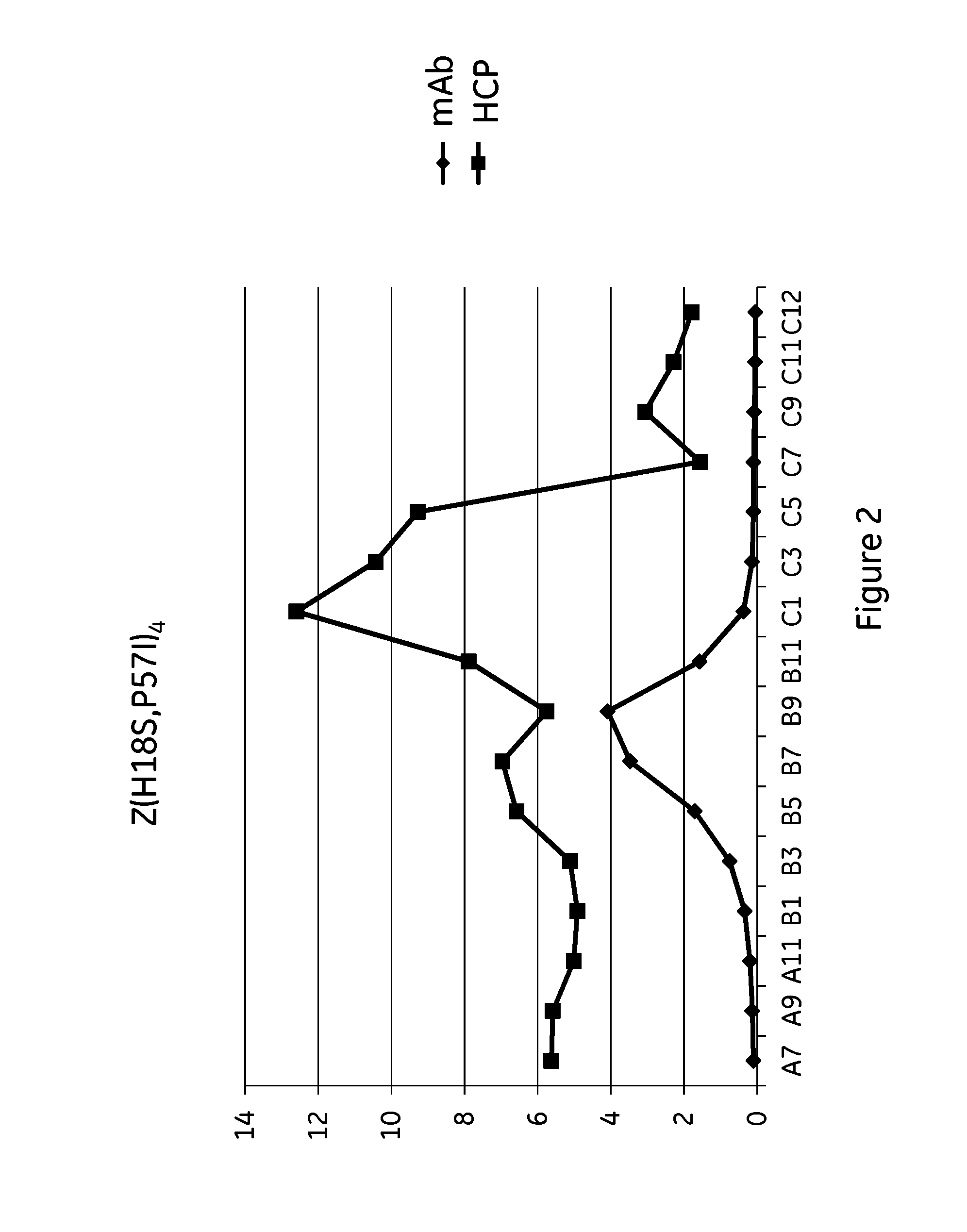
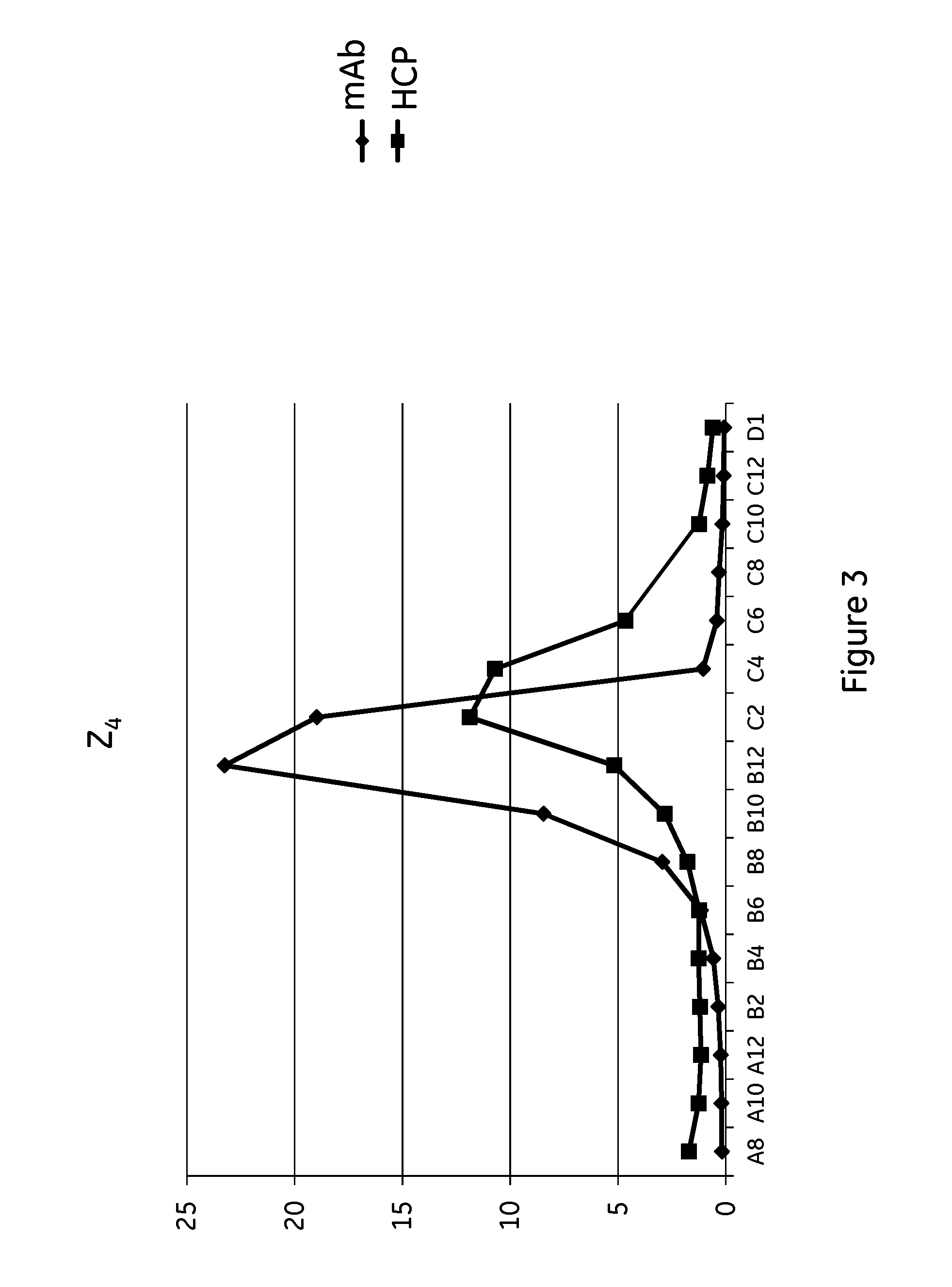
The invention discloses an immunoglobulin-binding protein comprising one or more mutated immunoglobulin-binding domains (monomers) of staphylococcal Protein A (E, D, A, B, C) or protein Z or a functional variant thereof, wherein in at least one of the one or more mutated monomers, the asparagine or histidine at the position corresponding to H18 of the B domain of Protein A or of Protein Z has been deleted or substituted with a first amino acid residue which is not proline or asparagine and wherein, if the amino acid residue at position 57 is proline and the amino acid residue at position 28 is asparagine, then the amino acid residue at the position corresponding to H18 of the B domain of protein A or of protein Z is not serine, threonine or lysine.
Owner:CYTIVA BIOPROCESS R&D AB
Performance improved recombination staphylococcus aureus protein A affinity ligand and construction method thereof
ActiveCN103214563AImprove bindingImprove elutionMicroorganism based processesDepsipeptidesHigh concentrationEscherichia coli
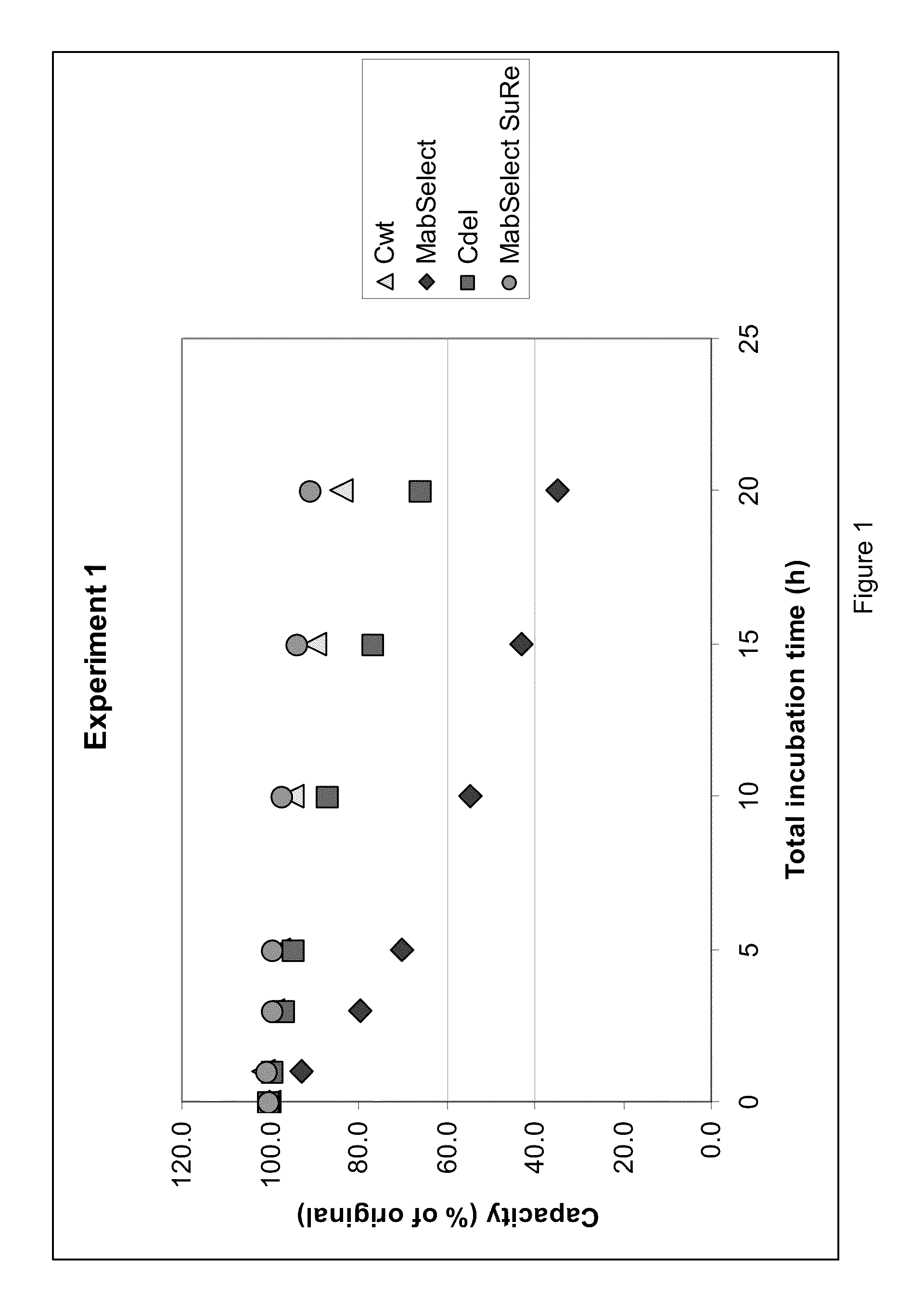
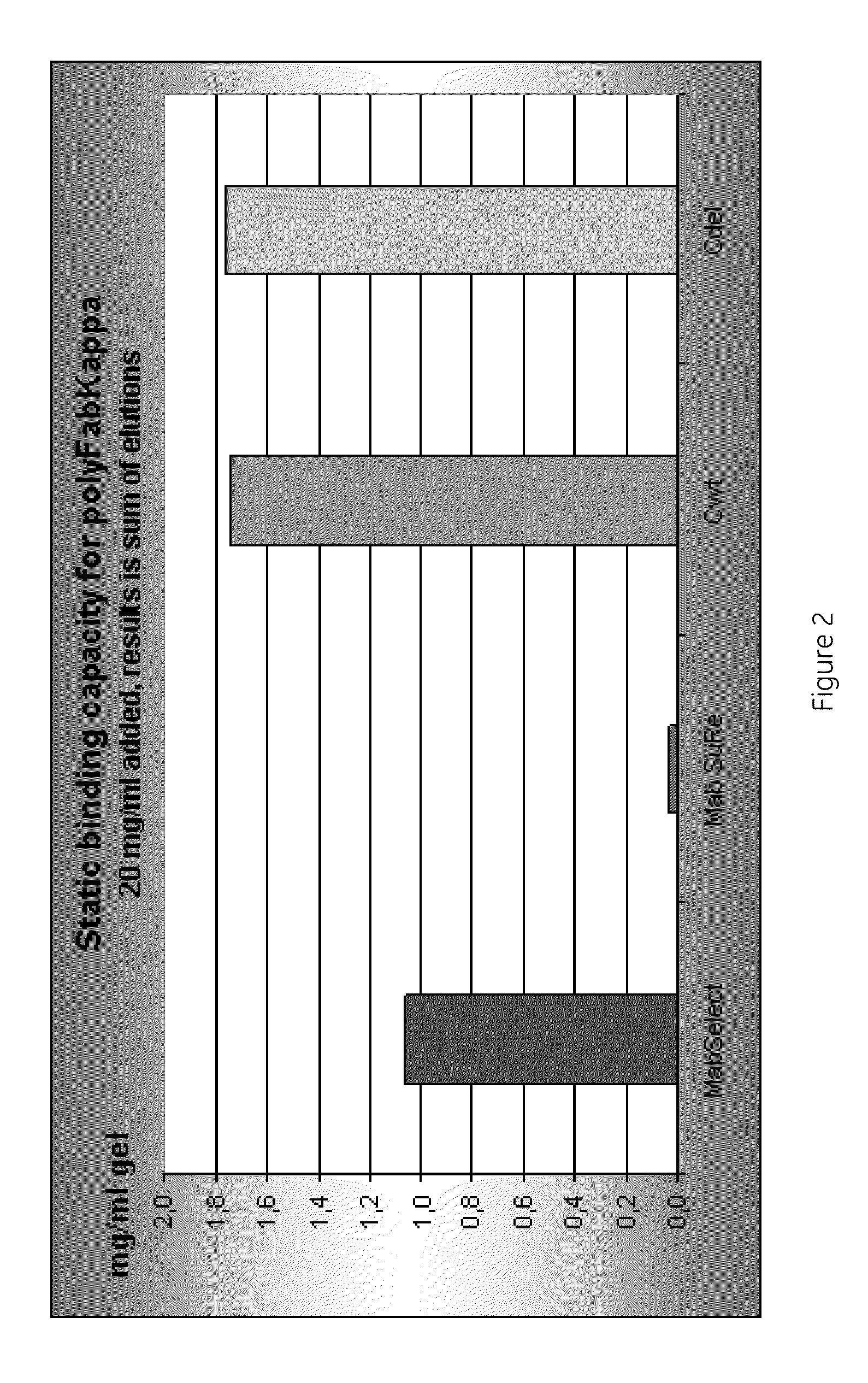
The present invention discloses a performance improved recombination staphylococcus aureus protein A affinity ligand and a construction method thereof. The present invention adopts a molecular biology method. A sequence B of a nature protein A is selected for molecular transformation. A C-terminal of the sequence B is added with two cysteines, so that the protein A can pass through double-locus coupled chromatography matrix to stabilize the connection. Six glycines are added to the end of a second Loop of the sequence B to increase the length and reduce the binding force with an antibody, so that elution conditions are mild. On this basis, resistance performance to high concentration base of the protein A is transformed. Asparagines and phenylalanine at 23rd and 30th positions of the sequence B are respectively replaced by threonine and alanine to obtain a sequence Z with higher alkaline resistance properties. Then, isocaudarner is used for connecting sequence Zs of different numbers head-to-tail in series. The efficient expression system of e. coli is used for overexpression. The expressed recombination protein A is coupled to agarose matrix preparation affinity chromatography fillers and is used for purifying antibodies. Results show that the recombination protein A affinity ligand prepared by the present invention is good in elution performance and alkali resistance.
Owner:嘉兴千纯生物科技有限公司
Gene of recombinant staphylococal protein A, expression vector containing gene and application thereof
The invention discloses a gene of a recombinant staphylococal protein A and application thereof. The nucleotide sequence of the gene of the recombinant staphylococal protein A is shown as SEQ ID NO:1; and the amino acid sequence of the protein coded by the gene is shown as SEQ ID NO:2. The staphylococal protein A is fused with small ubiquitin to form the gene of the recombinant staphylococal protein A; the expressed recombinant protein facilitates purifying the protein and improves the stability and activity of the protein; and experiments prove that the bioactivity of the expressed recombinant staphylococal protein A is not obviously different from that of a natural staphylococal protein A. The recombinant staphylococal protein expression and purification method has the advantages of short production time, high expression efficiency, high expression level, simple purification and the like.
Owner:JIANGSU KANIONREAL BIOMEDICAL TECH CO LTD
Multiple index quick detecting method for human immune defect virus antibody
InactiveCN1858594ASimultaneous diagnosis and differentiation of infectionSimple and fast operationMaterial analysis by observing effect on chemical indicatorHuman immunodeficiency virus antibodyEscherichia coli
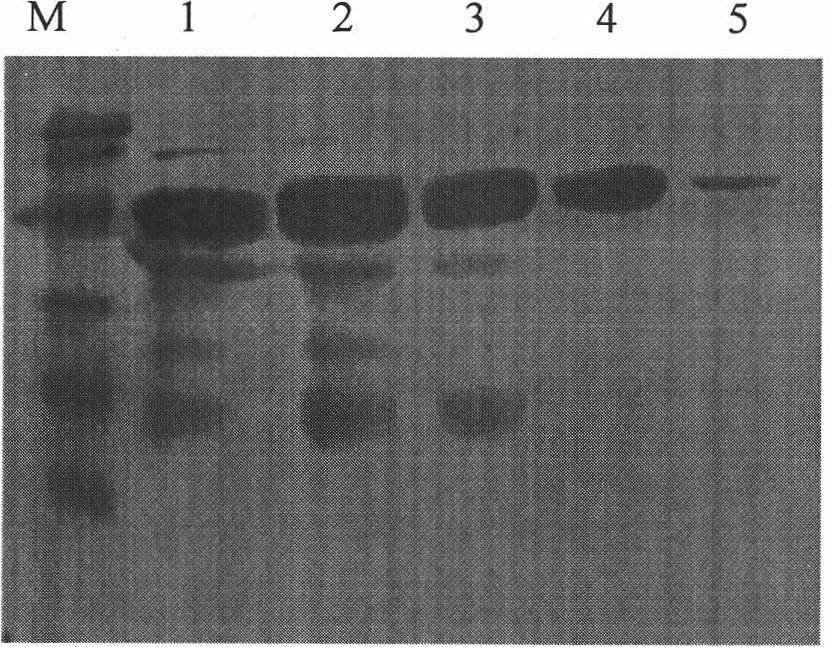
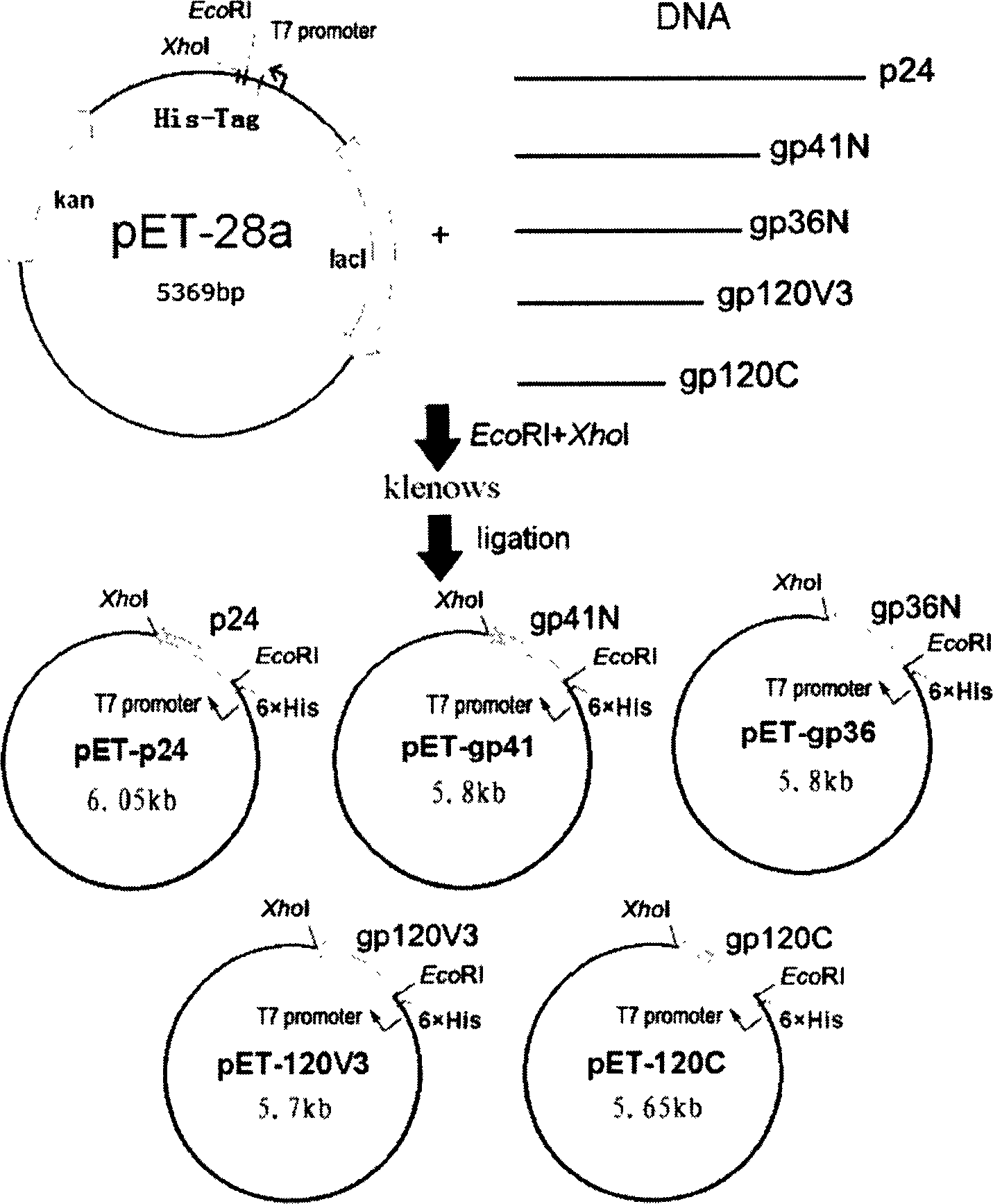
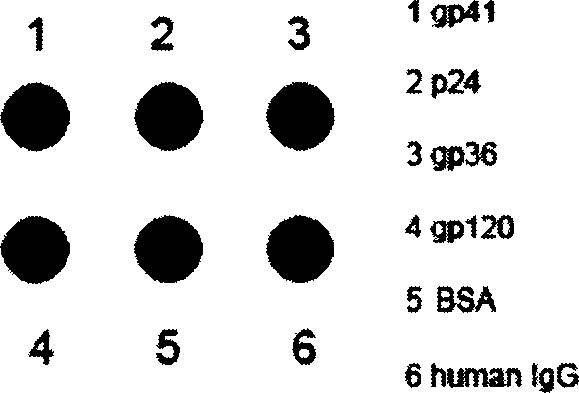
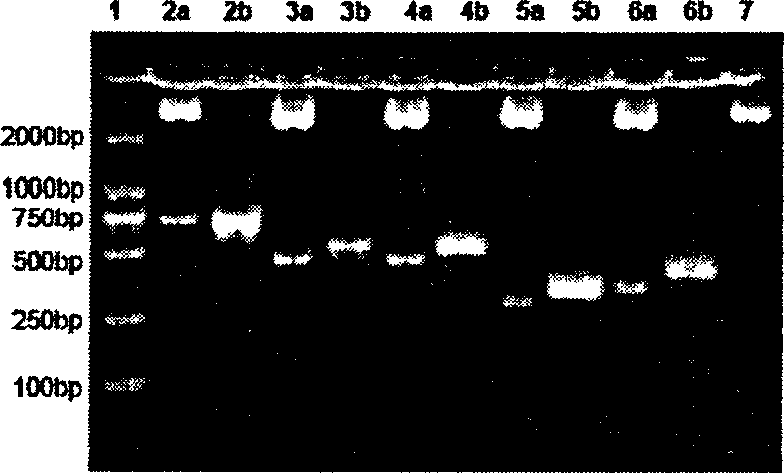
This invention discloses a multi-target quick test method for human immunodeficiency virus antibodies including: 1, expressing five kinds of HIV antigen white dribs and drabs-p24, gp41, gp36, gp120V3, gp120C in a colibacillus by a gene engineering technology, 2, fixing the five antigen whites on a pyroxylin film, 3, dripping armed serum on it and the virus antibody is combined with the antigen by a immunoreaction then adding the A(SPA) labeled by nm gold, 4, cleaning it after it penetrates the film, 5, adding SPA antibodies to increase the amplification to form red spots seen by eyes.
Owner:WUHAN UNIV
Mutated immunoglobulin-binding polypeptides
ActiveUS9663558B2Improve stabilityHighly selectiveOther chemical processesSolid sorbent liquid separationStaphylococcusImmunoglobulin IgE
A polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A, as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, wherein at least the glutamine residue at position 15 has been mutated to an amino acid other than asparagine. The invention also discloses multimers of the polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
Immunofluorescence label reagent kit
InactiveCN101149372ASimple and fast operationImprove stabilityBiological testingFluorescenceImmunofluorescent labeling
This invention discloses to a kind of immunofluorescence mark reagent box. It is a reagent box applied for the aspects of immunity mark which uses the green fluorescence protein (GFP) as the indicator, the staphylococcus protein A (SPA) as the antibody. It also discloses the preparation method of the reagent. This invention can applied wide range in the field such as the immunity fluorescence mark, immunity histochemistry, biochemistry analysis, antibody test, protein chip and so on. It can replace the exist product efficiently, its operation is more simple, stability, delicacy and reliability, it is especially the same with the high flux analysis which analyze by the fluorescence analysis instrument.
Owner:SHANDONG UNIV AT WEIHAI
Human-outer autoantibody radio-immunity quantitative determination method
InactiveCN101256189ARapid quantitative detection technology platformBiological testingQuantitative determinationAutoantibody production
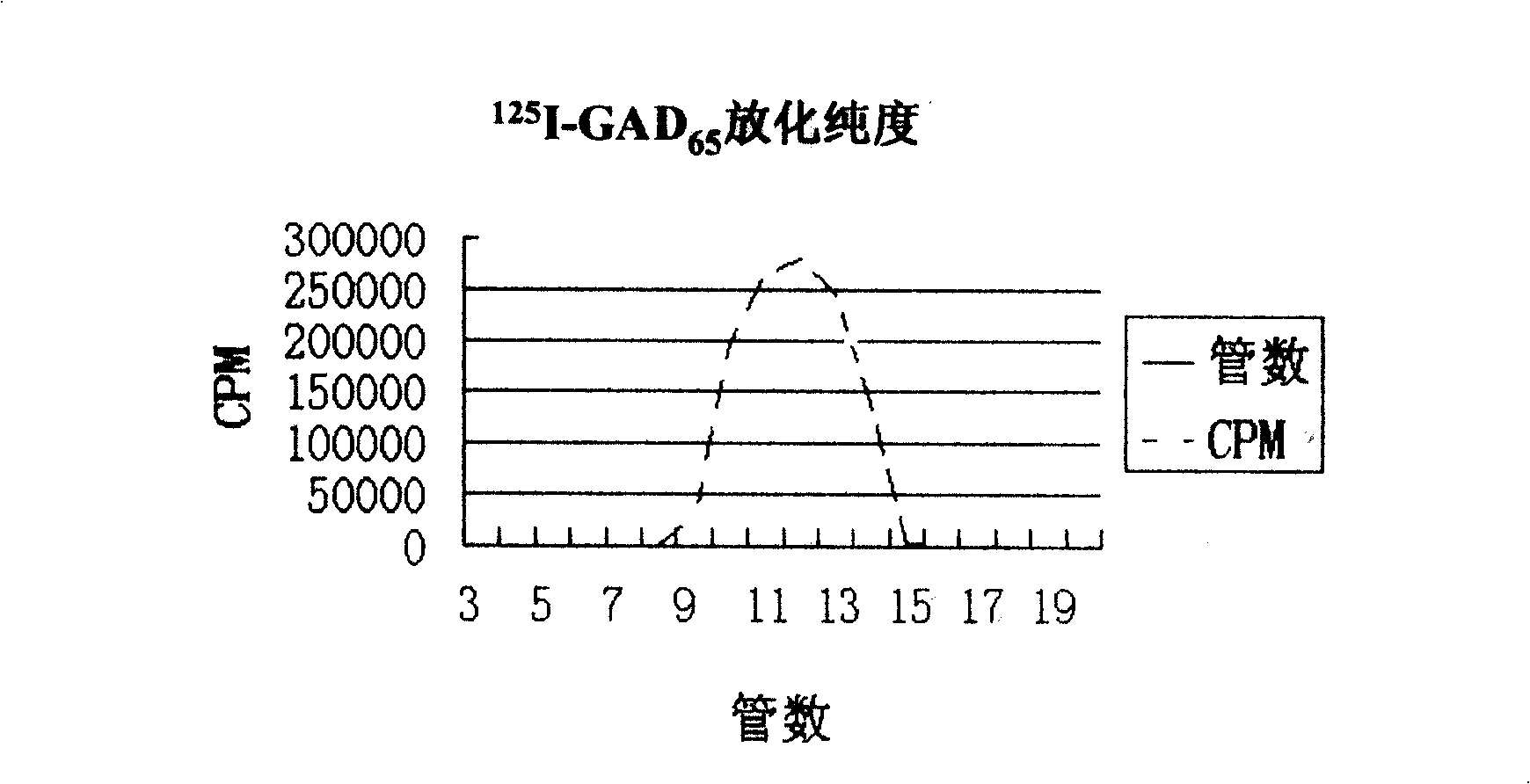
The invention relates to a quantificationally detecting method for autoantibody in vitro, belonging to biomedicine technical field. The general character that the staphylococcal bacteria protein A (SPA) can react with Fc site of IgG in human and mouse is skillfully used and monoclonal antibodies of high affinity and specificity are prepared into standard substance to establish standard curve, therefore the monoclonal antibodied and autoantibody in sample respectively react with the labelled antigens, and the SPA enveloped nm magnetic grains are used as ligands to perform solid-liquid separation, after radioactivity measurement of 125I, finally the radioactive ligand immunity quantitative analysis method of autoantibody is established. The said method is suitable for quantificationally detecting the autoantibody, so as to solve the problem that most of the antoantibody can not be accurately and quantificationally detected and at the same time prevent cross reaction without false negative or false positive phenomena.
Owner:天津市协和医药科技集团有限公司
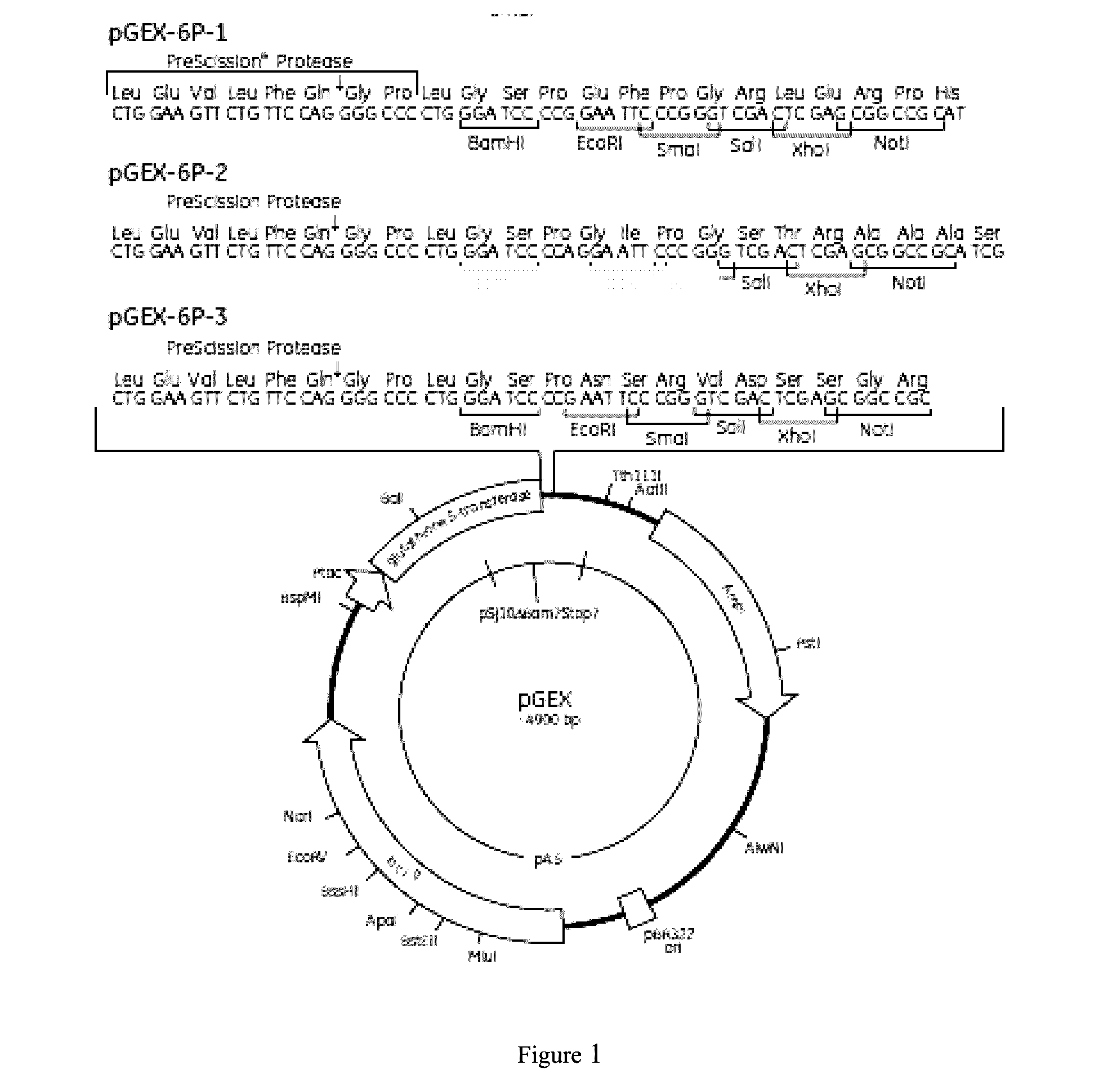
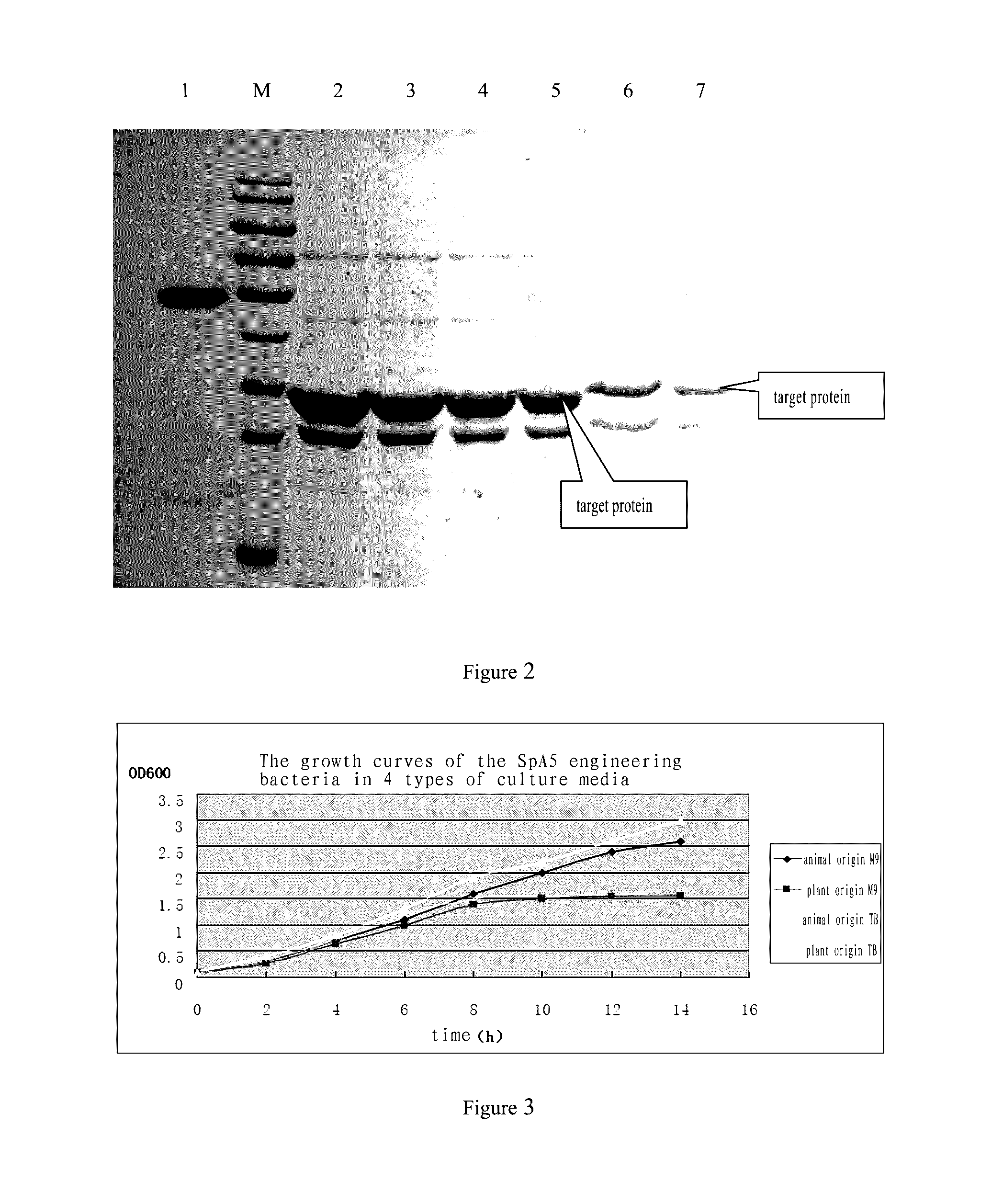
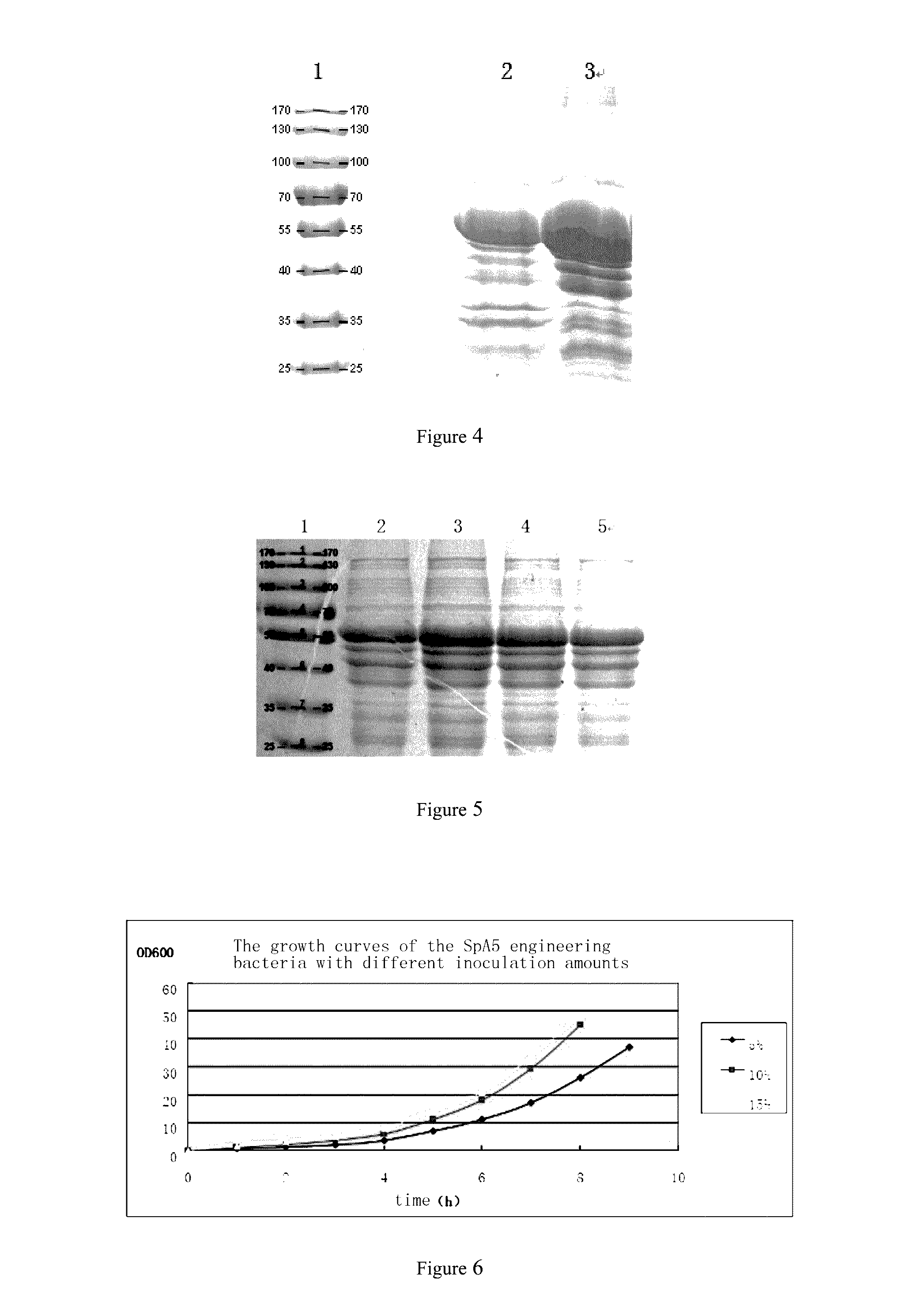
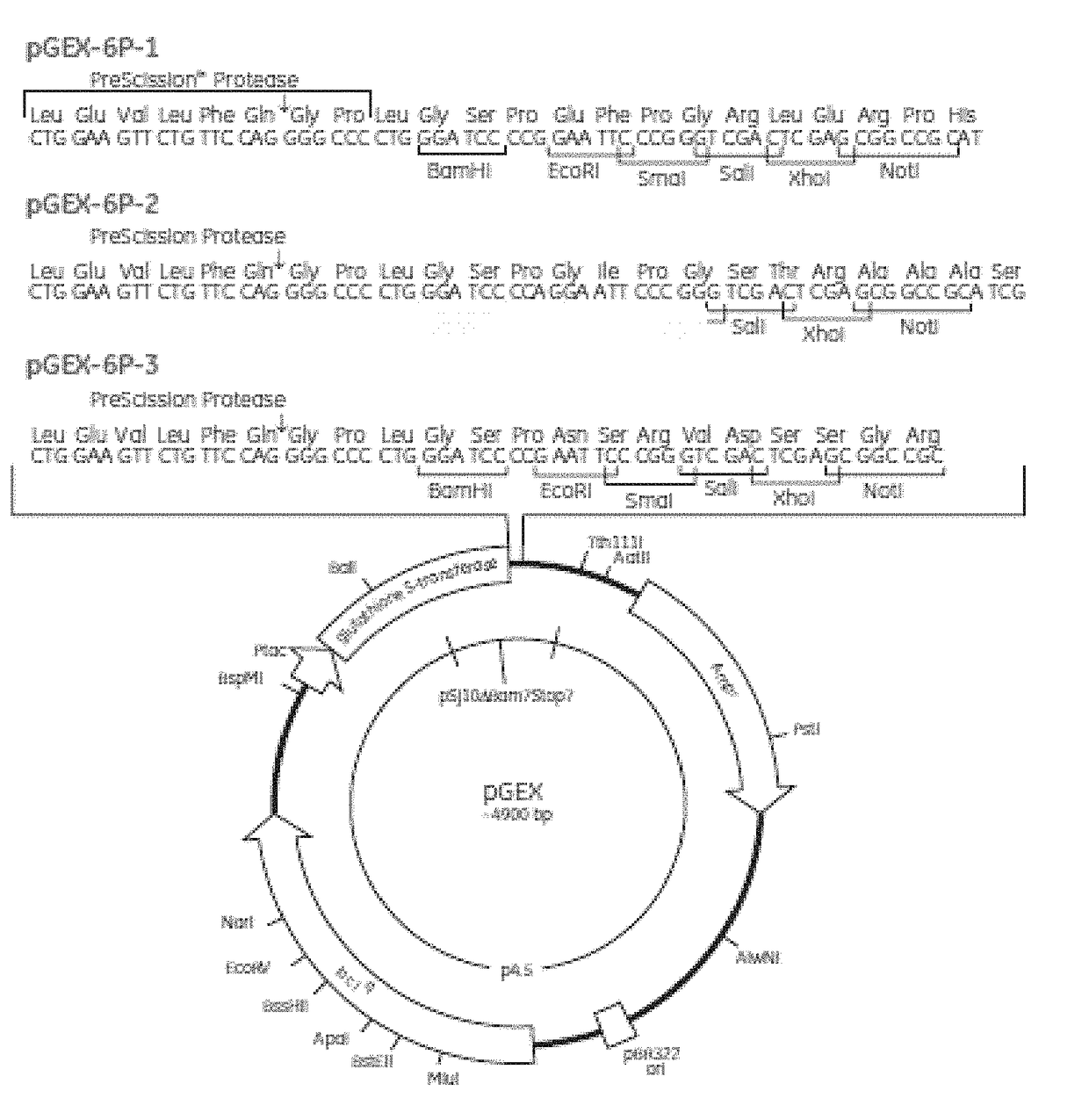
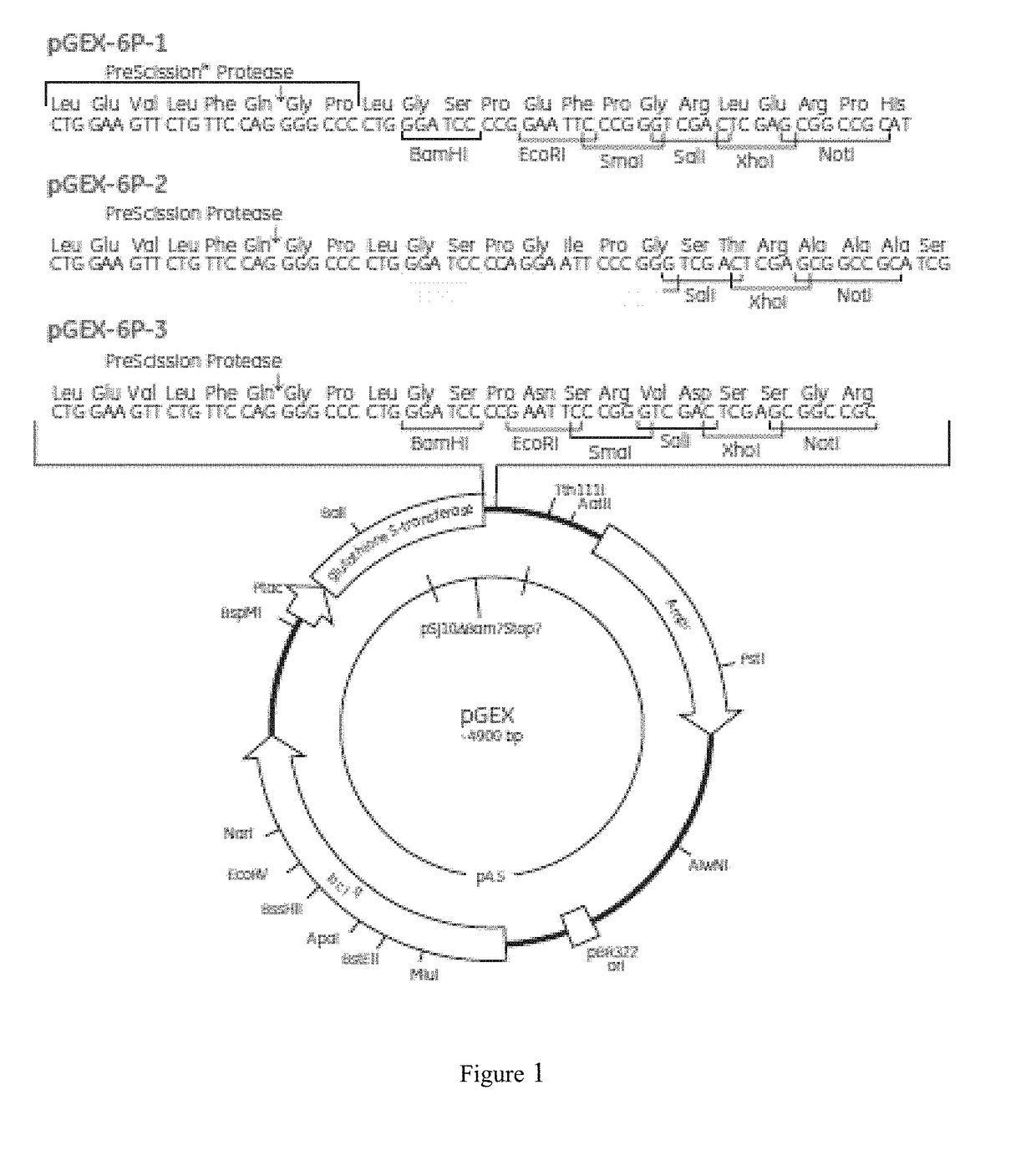
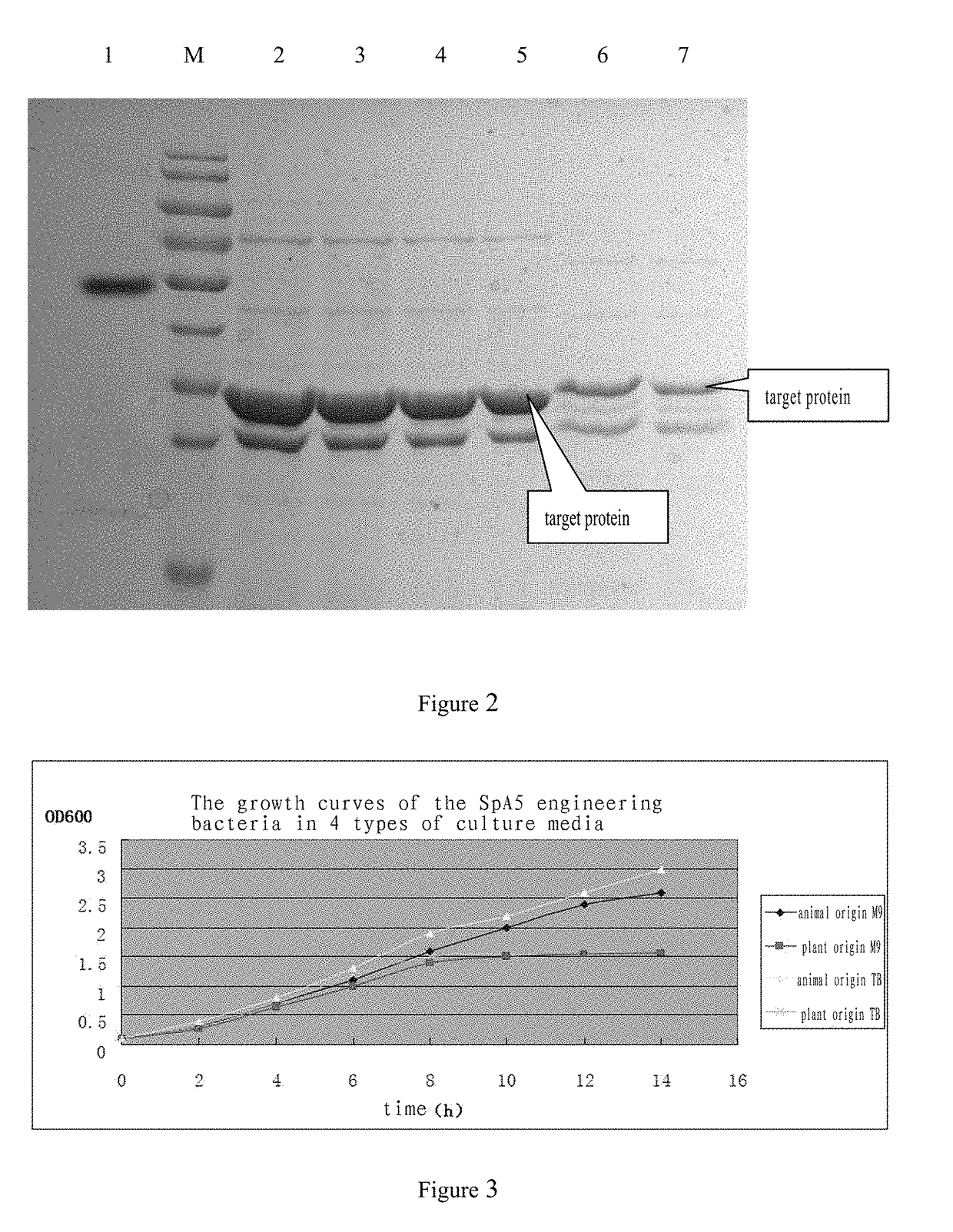
Staphylococcus aureus spa5 mutant, composition comprising mutant and preparation method and use thereof
ActiveUS20160304566A1Protective immune responseImproving immunogenicityAntibacterial agentsAntibody mimetics/scaffoldsBacteroidesStaphylococcus cohnii
Provided is staphylococcus protein A expressed by a mutational Staphylococcus aureus and its coding sequence, as well as a vector, host bacteria, composition or kit which contains the coding sequence of the mutational protein. Also provided is the use of the mutational protein and the composition thereof in the preparation of vaccines, therapeutic antibodies, diagnostic kits and the like, and for the prevention, treatment and detection of infections by Staphylococcus aureus. Also provided are methods for producing, fermenting and purifying the mutational protein.
Owner:CHONGQING YUANLUN BIOTECH +1
Staphylococcus aureus SpA5 mutant, composition comprising mutant and preparation method and use thereof
ActiveUS9890199B2Protective immune responseEfficient triggerAntibacterial agentsAntibody mimetics/scaffoldsBacteroidesStaphylococcus cohnii
Provided is staphylococcus protein A expressed by a mutational Staphylococcus aureus and its coding sequence, as well as a vector, host bacteria, composition or kit which contains the coding sequence of the mutational protein. Also provided is the use of the mutational protein and the composition thereof in the preparation of vaccines, therapeutic antibodies, diagnostic kits and the like, and for the prevention, treatment and detection of infections by Staphylococcus aureus. Also provided are methods for producing, fermenting and purifying the mutational protein.
Owner:CHONGQING YUANLUN BIOTECH +1
Anti-bacterial antibodies
InactiveUS8142780B2Efficient infectionFacilitate immune system-mediated clearanceAntibacterial agentsAntibody ingredientsMonoclonal antibodyAntigen binding
A pharmaceutical composition includes a purified antibody and a pharmaceutically acceptable carrier. The antibody can be a monoclonal antibody having both an antigen-binding portion that binds at least one bacterial antigen and a constant region that does not bind staphylococcal protein A.
Owner:STROX BIOPHARMLS
Mutated immunoglobulin-binding polypeptides
ActiveUS20160207966A1Improve stabilityHighly selectivePeptide/protein ingredientsSolid sorbent liquid separationMutantGlutamine
A polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A, as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, wherein at least the glutamine residue at position 15 has been mutated to an amino acid other than asparagine. The invention also discloses multimers of the polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
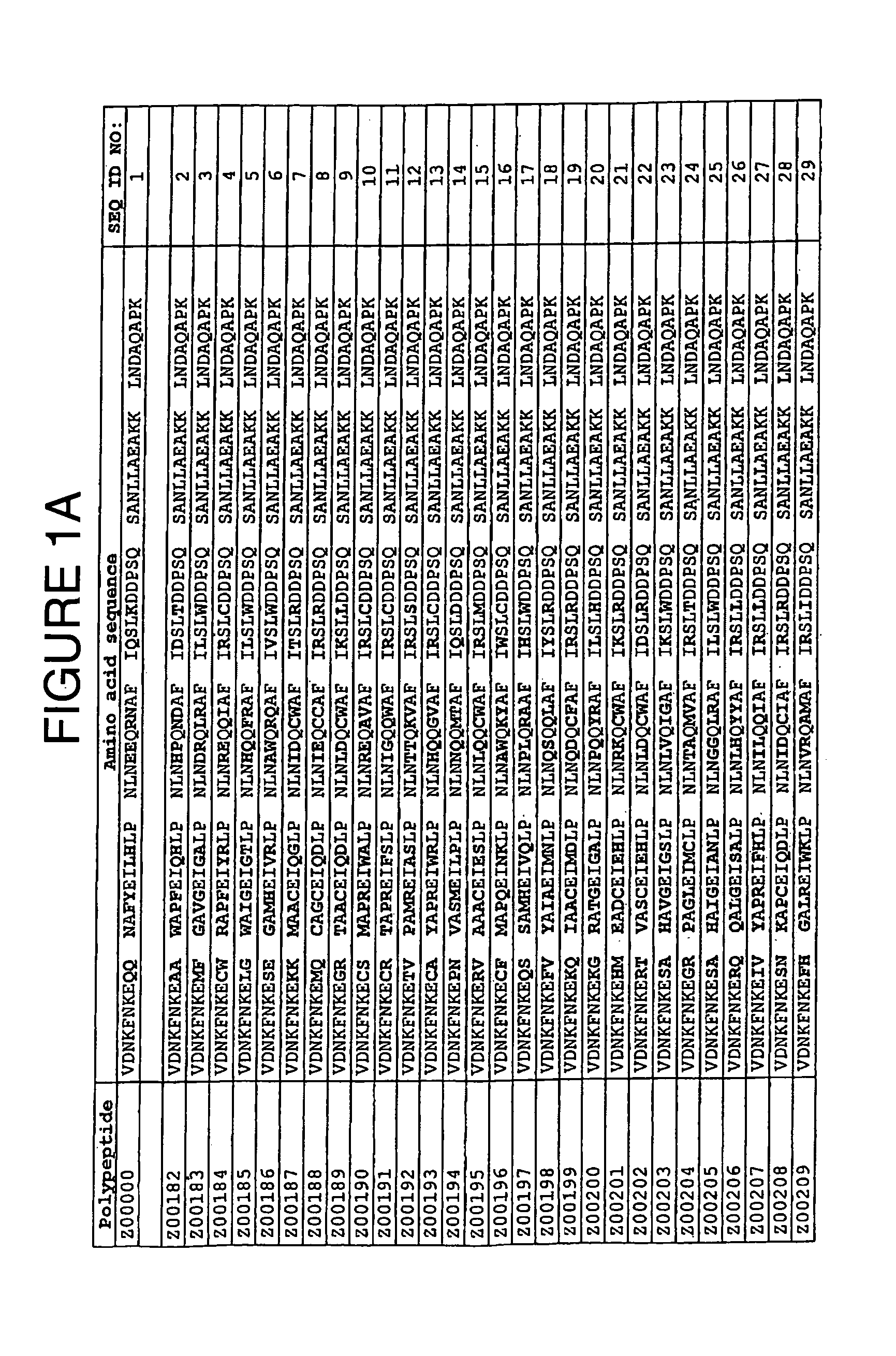
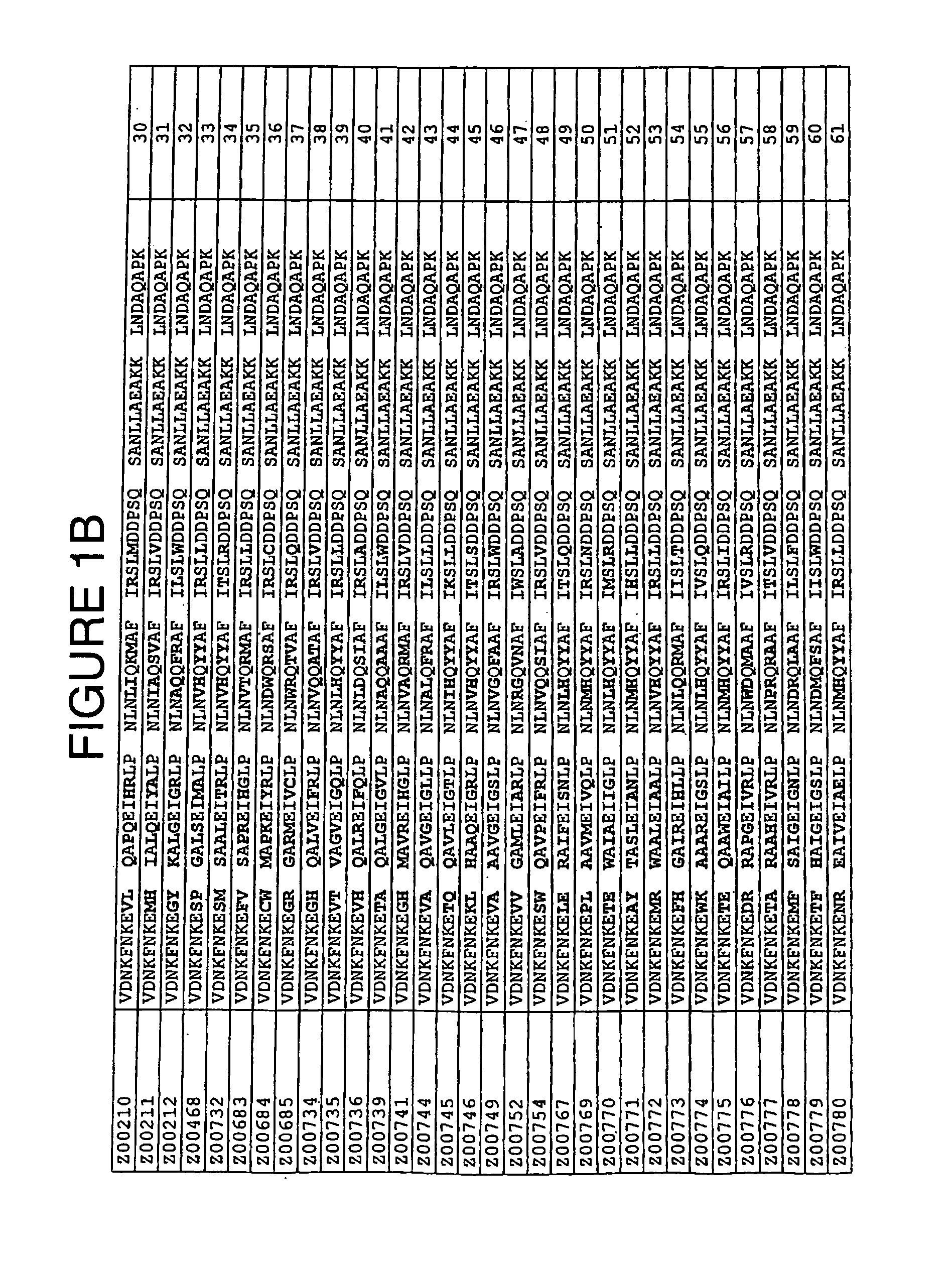
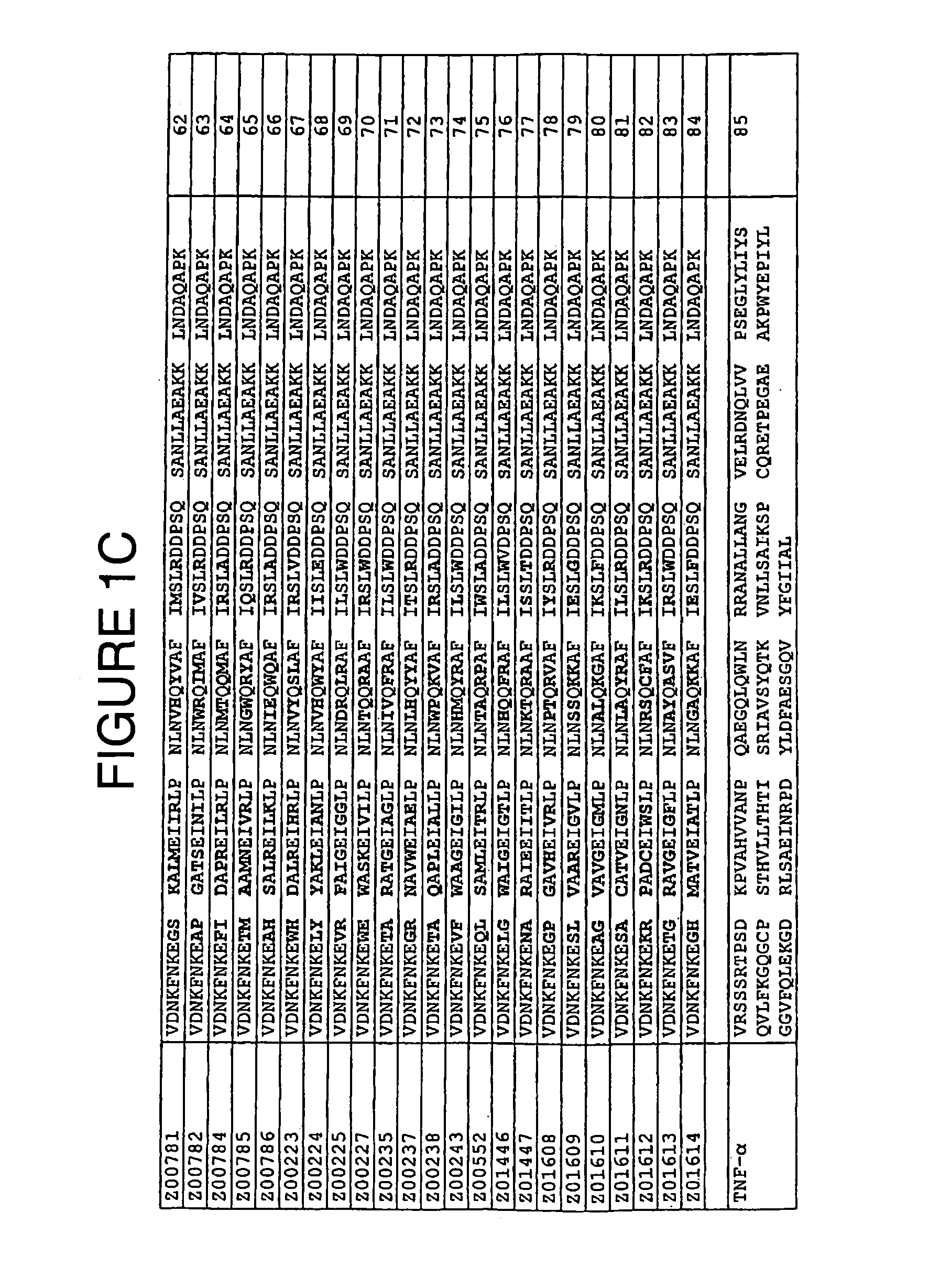
Staphylococcus protein A domain mutants that bind to TNF-α
InactiveUS7993848B2Exclude false positive resultsSugar derivativesPeptide/protein ingredientsA domainBody fluid
A TNF-α binding polypeptide is provided, which is related to a domain of staphylococcal protein A (SPA) in that the sequence of the polypeptide corresponds to the sequence of the SPA domain having 1 to about 20 substitution mutations. Nucleic acid encoding the polypeptide, expression vector comprising the nucleic acid, and host cell comprising the expression vector are also provided. Also provided are methods comprising a step of affinity separation or detection, in which step a polypeptide according to the invention is used. Such methods may be used for reducing the content of TNF-α in a body fluid.
Owner:AFFIBODY TECH AB
Method of separating and purifying recombinant stphylococcl protein A
ActiveCN106977591AHigh yieldHigh purityDepsipeptidesPeptide preparation methodsEscherichia coliProtein solution
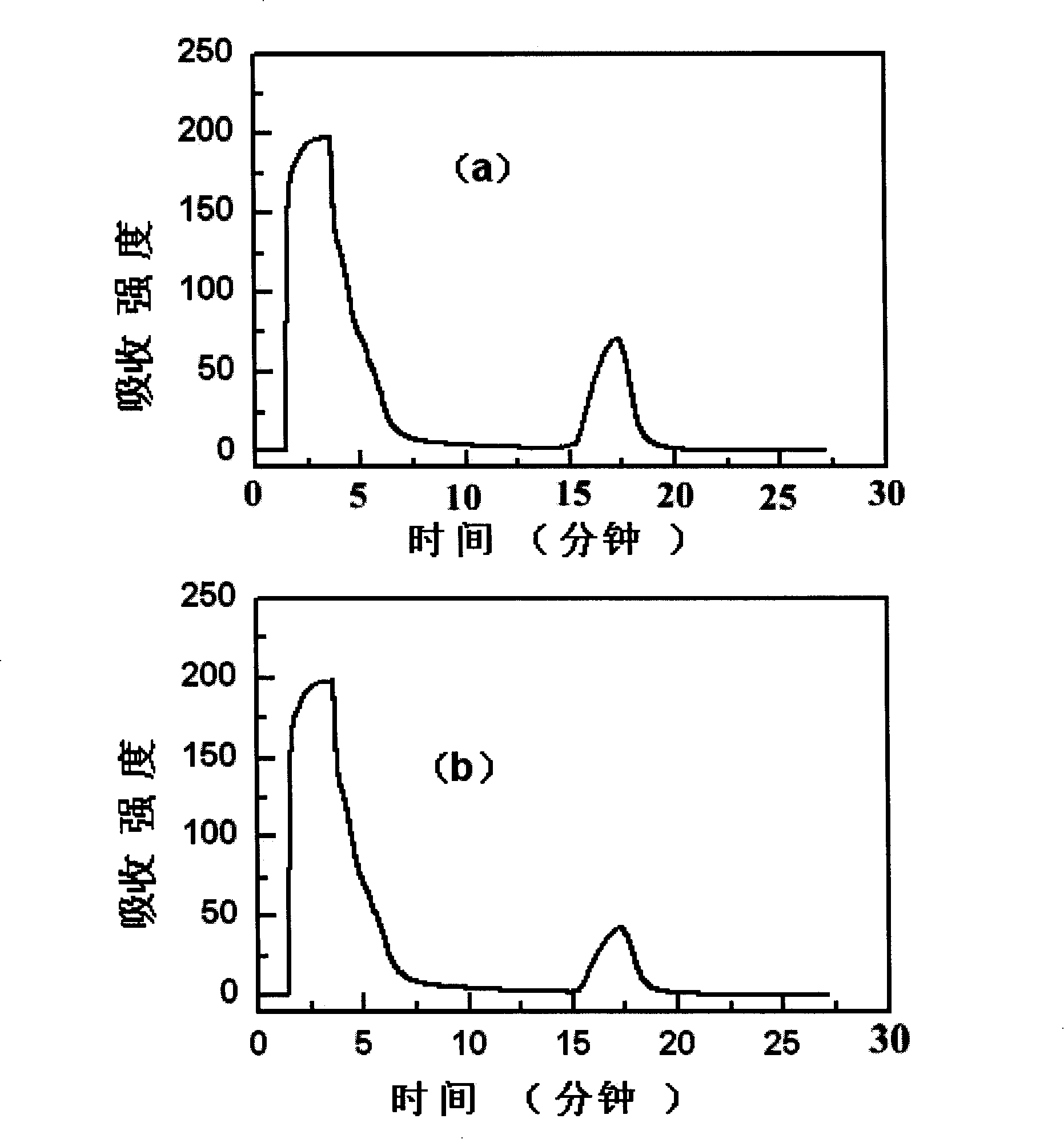
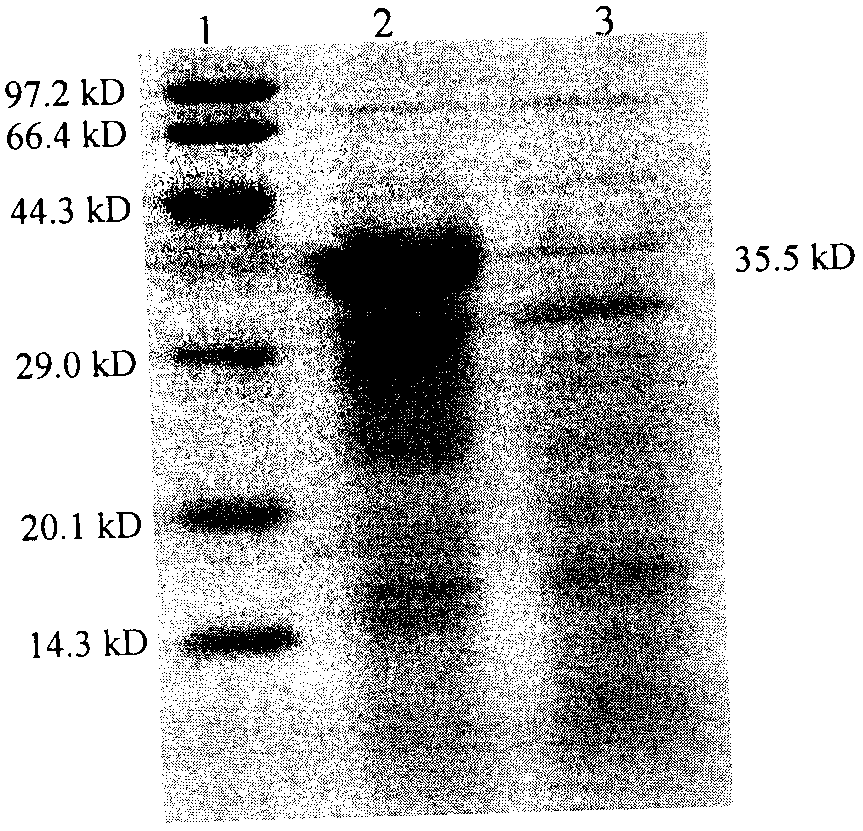
The invention discloses a method of separating and purifying recombinant stphylococcl protein A. A series of impurities such as endotoxin, residual proteins (HCP) of escherichia coli, residual DNA escherichia coli and protein aggregates are effectively removed by adopting stuffing of two mixing mechanisms by way of two-step chromatography and ultrafiltration. The purest target sample is obtained by fewest steps, so that the process control cost is lowered, and the method has the characteristics of being good in stability, clear in process control index, suitable for scaled industrial production the like. The protein purity of a purified protein solution is greater than 97% by means of high performance liquid and SDS-PAGE electrophoresis; the protein recovery rate is greater than 75%; the endotoxin is reduced to 1EU / mg below; HCP and residual DNA of escherichia coli are lower than 5ppm, and the recombinant stphylococcl protein A can be used as a raw material for synthesizing clinical staphylococcus protein A immunosorbent.
Owner:GUANGZHOU KONCEN BIOSCI
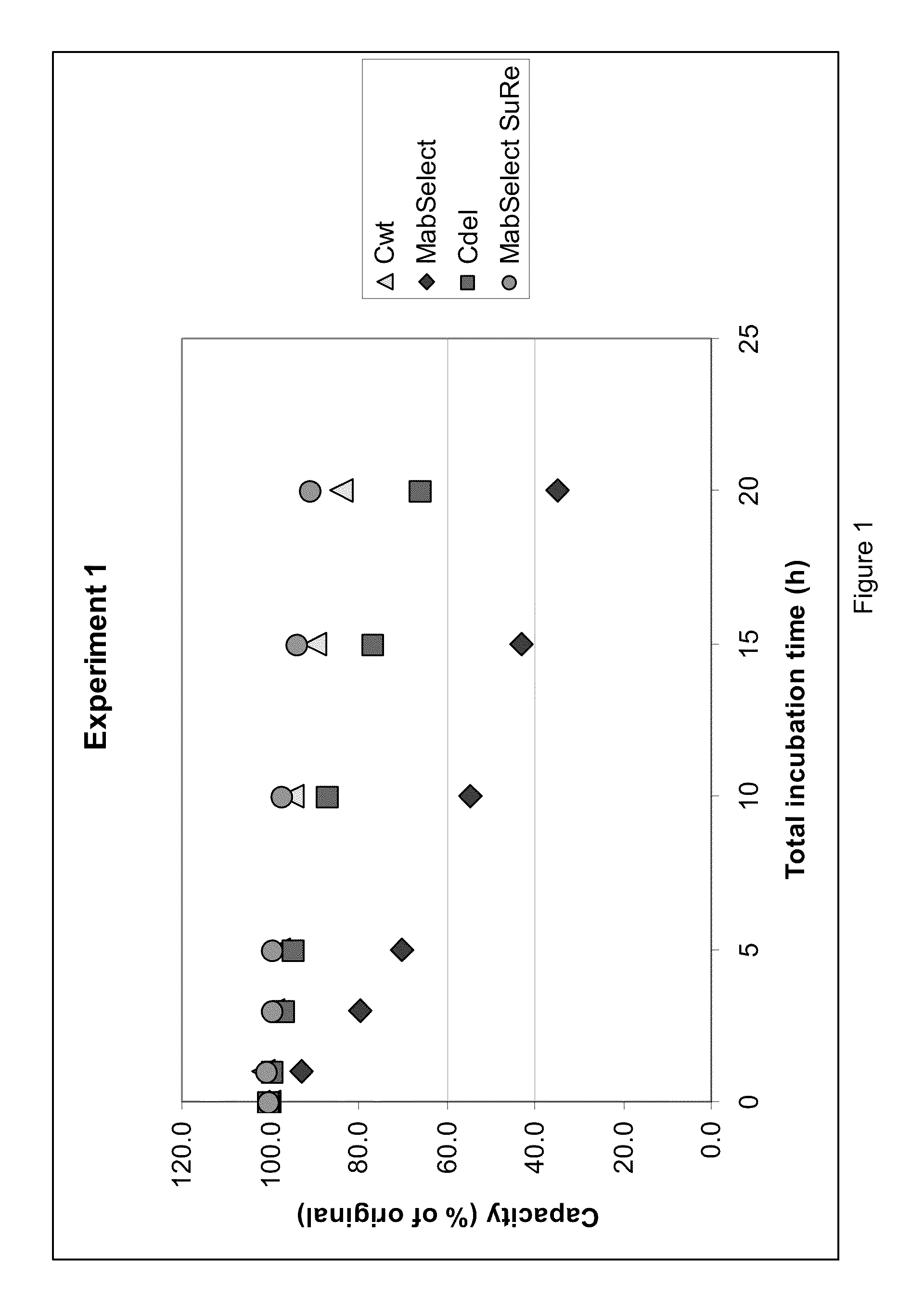
Caustic stable chromatography ligands
ActiveUS10072050B2Cost-effectiveComponent separationOther chemical processesChromatography columnImmunoglobulin binding
The present invention relates to chromatography ligands having improved caustic stability, e.g., ligands based on immunoglobulin-binding proteins such as, Staphylococcal protein A, as well as methods of making and using such ligands.
Owner:MILLIPORE CORP
Quantum dot fluorescence chromatography immunization analysis reagent for detecting human blood ZnT8A
The invention discloses a quantum dot fluorescence chromatography immunization analysis reagent for detecting human blood ZnT8A. The reagent comprises a base plate, a sample pad, a quantum dot-labeledcombination pad, a chromatography reaction membrane and a water absorption pad are arranged at the upper surface of the base plate, a quantum dot-labeled mouse-anti-human IgG antibody is absorbed atthe quantum dot-labeled combination pad, a detection line fixed with ZnT8 protein and a quality control line fixed with staphylococcal protein A are provided at the chromatography reaction membrane, to-be-detected human whole blood or a blood plasma sample are dropped to the sample pad, and are subjected to a chromatography reaction for 3-4 min, a fluorescence immunoassay analyzer is used for detecting the fluorescence intensity of the detection line and the quality control line, and the zinc transportprotein 8 self-antibody content in a sample can be calculated by built-in software. The reagent has the advantages of simple operation, fast reaction, high sensitivity, and strong specificity.
Owner:天津市协和医药科技集团有限公司 +1
Anti-bacterial antibodies
InactiveUS20100322944A1Efficient infectionFacilitate immune system-mediated clearanceAntibacterial agentsAntibody ingredientsMonoclonal antibodyAntigen binding
A pharmaceutical composition includes a purified antibody and a pharmaceutically acceptable carrier. The antibody can be a monoclonal antibody having both an antigen-binding portion that binds at least one bacterial antigen and a constant region that does not bind staphylococcal protein A.
Owner:STROX BIOPHARMLS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com