Human-outer autoantibody radio-immunity quantitative determination method
A quantitative detection method and autoantibody technology, applied in the field of biomedicine, can solve problems such as inducing diseases and physical damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The content of this method is specified by the following examples:
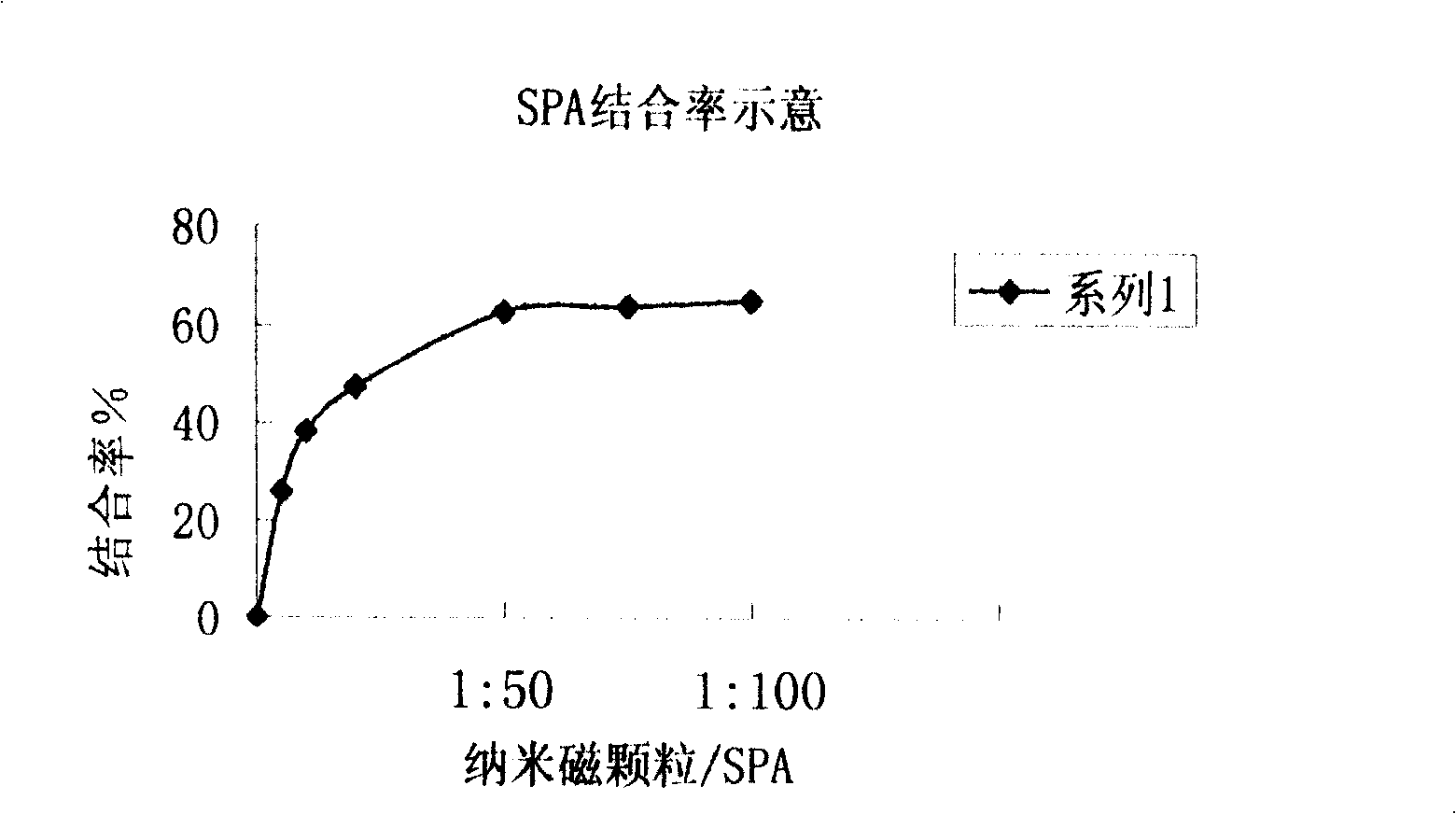
[0022] GAD 65 Establishment of Quantitative Analysis Method for Autoantibody Chemiluminescence Ligand (Nanomagnetic Particles)
[0023] (1) Preparation of each component of the kit:
[0024] 1. Labeled antigen ( 125 I-GAD 65 ) preparation
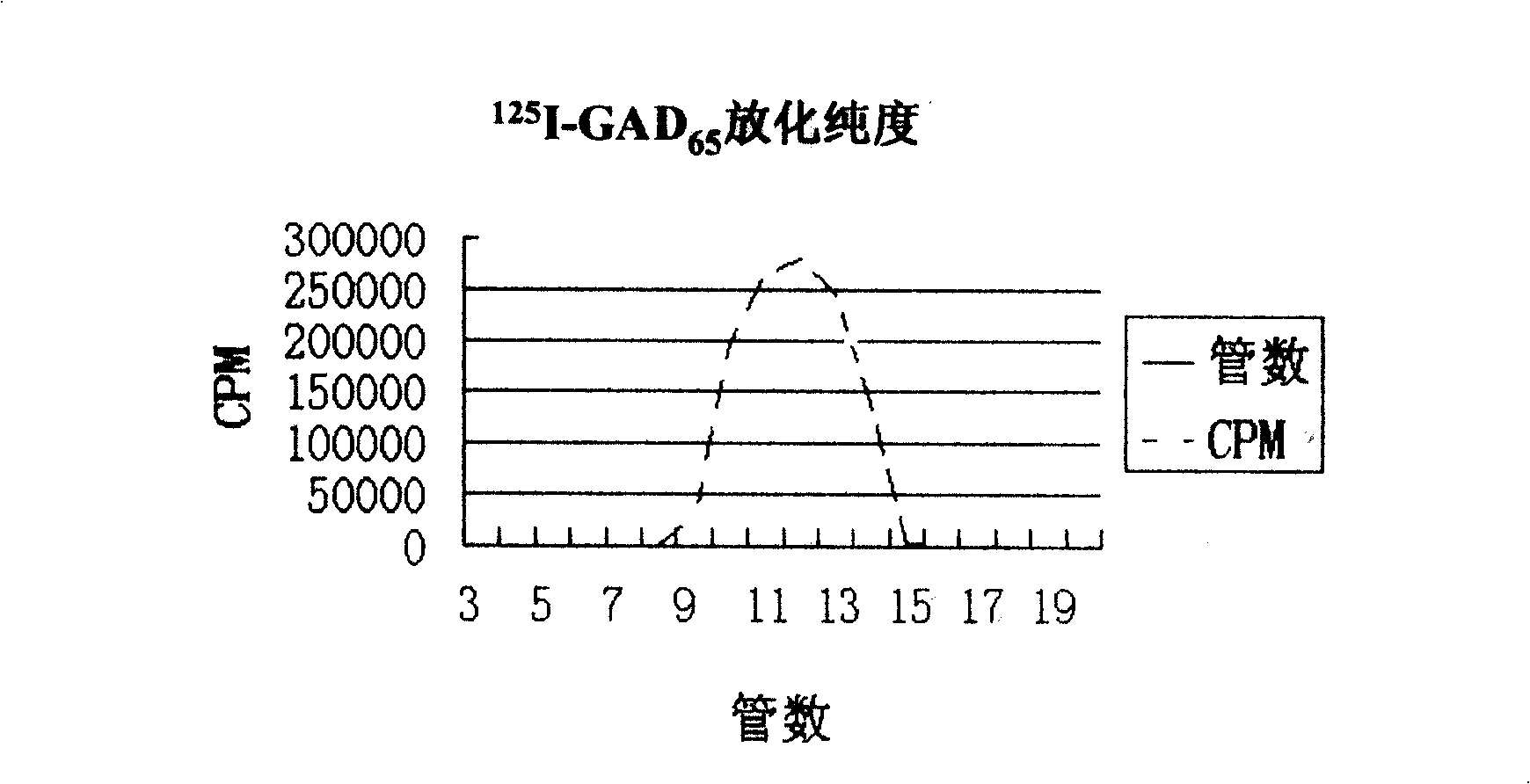
[0025] (1) Prepared by improved lactoperoxidase labeling technology 125 I-GAD 65 :
[0026] GAD 65 10~50μg dissolved in 50~200μl of pH6.5 phosphate buffer, add Na 125 I 1~8mCi, lactoperoxidase solution 40~150ng (10~50μl), hydrogen peroxide (H 2 o 2 )400~800ng (50~100μl), during incubation at 37℃, add H dropwise 2 o 2 400~800ng (50~100μl), complete within 5~10 minutes, continue the reaction for 2~5 minutes, add 0.5~2.0ml 20mmol / L mercaptoethanol for 30~60 seconds to terminate the reaction, and quickly transfer the reaction solution to the pre-used Chromatographic separation on Sephadex G50 or Sephadex G25 column equilibrated with pH 6.5-7.5 phosphate buf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com