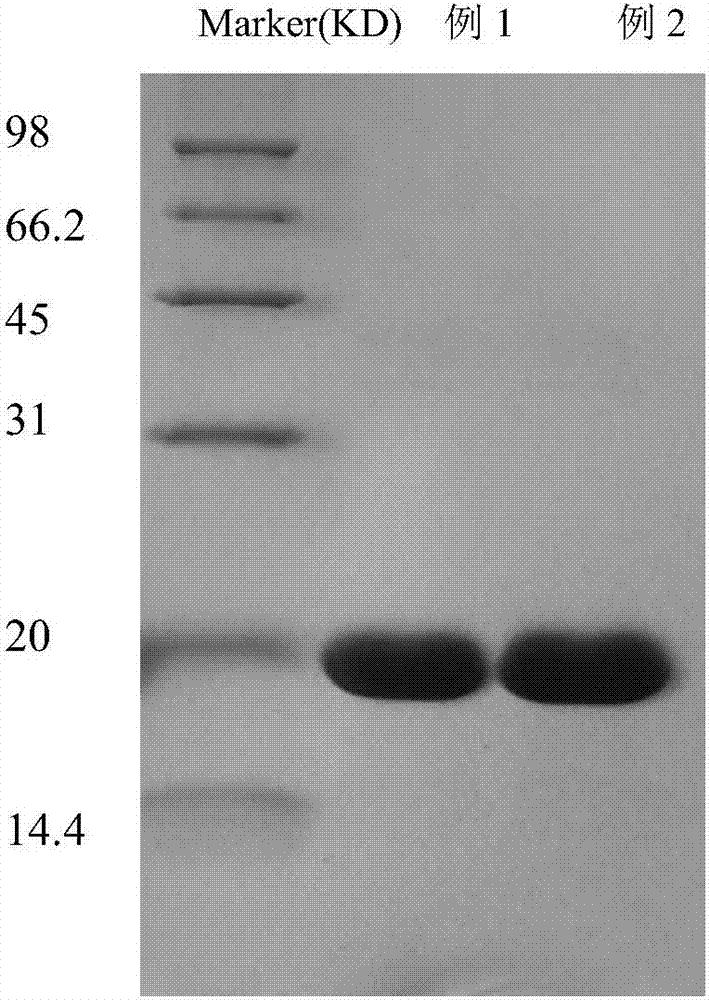
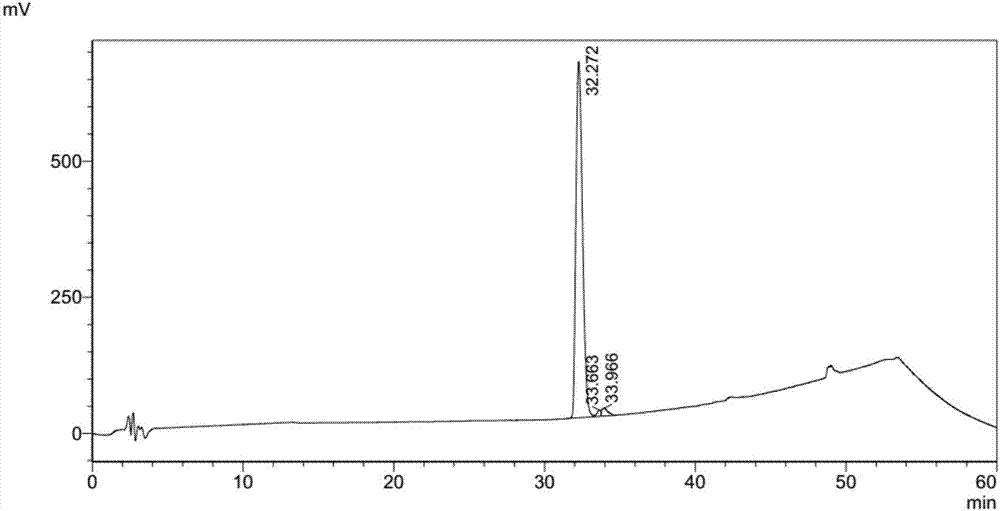
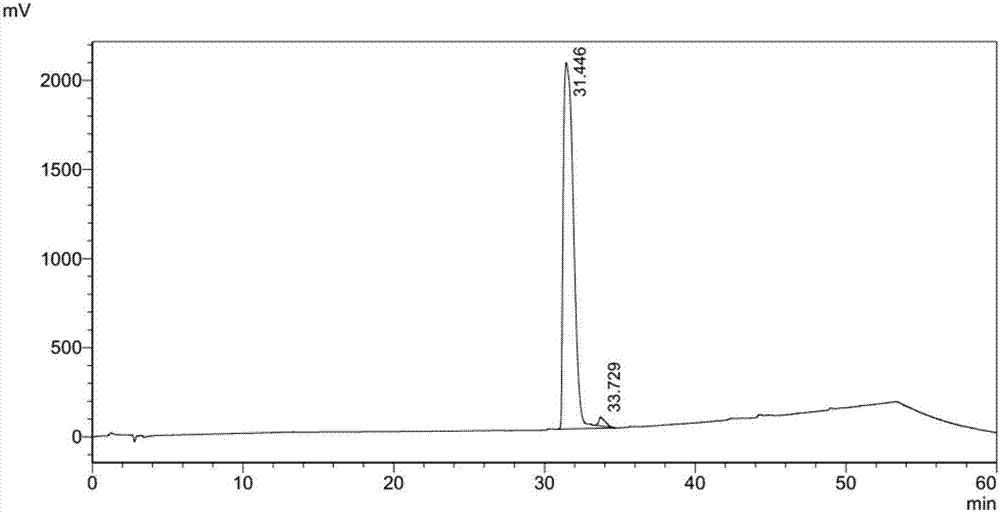
Method of separating and purifying recombinant stphylococcl protein A
A staphylococcal protein, separation and purification technology, applied in the field of separation and purification of recombinant staphylococcal protein A, can solve the problems of inconvenient operation, etc., and achieve the effect of reducing residual amount, reducing purification pressure, and combining tightly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Cell pretreatment: take 1 kg of cells containing staphylococcal protein A (such as genetically engineered bacteria E. coli expressing staphylococcal protein A), add 10 L of Bis-tris buffer solution with a pH=7.2 and a concentration of 10 mM to suspend the bacteria The body was crushed twice with a high-pressure homogenizer at a pressure of 800 bar, and about 10 L of the supernatant was collected by centrifugation. The resulting solution was added with hydrochloric acid to adjust the pH to 2.0, centrifuged, and the supernatant was adjusted to pH = 6.5 with sodium hydroxide to obtain a protein solution.
[0041] 2) The first chromatographic column treatment: will be equipped with TOYOPEARL NH 2 The chromatographic column with -750F filler is equilibrated with 5 times the column volume with an equilibrium solution containing 10mM Bis-tris and 0.5wt% TritonX-100, 0.1mol / LNaCl, pH 6.5, and the protein solution obtained in step 1 is loaded onto the used TOYOPEARL NH equil...
Embodiment 2
[0049] 1) Bacteria pretreatment: take 1 kg of bacteria containing staphylococcal protein A (such as genetically engineered bacteria Escherichia coli expressing staphylococcal protein A), add 10 L of Bis-tris buffer solution with a pH=7.2 and a concentration of 30 mM to suspend the bacteria The body was crushed twice with a high-pressure homogenizer at a pressure of 800 bar, and about 10 L of the supernatant was collected by centrifugation. The resulting solution was added with hydrochloric acid to adjust the pH to 4.0, centrifuged, and the supernatant was adjusted to pH = 7.2 with sodium hydroxide to obtain a protein solution.
[0050] 2) The first chromatographic column treatment: will be equipped with TOYOPEARL NH 2 Equilibrate 5 times column volume with equilibrium solution (containing 20mM Bis-tris and 1wt% TritonX-100, 0.1mol / LNaCl, pH 7.0) for the chromatography column with -750F filler, and load the protein solution obtained in step 1 to the used equilibrium Liquid bal...
Embodiment 3
[0058] Basically the same as in Example 1, the difference is that step 1) cell pretreatment: take 1 kg of cell containing staphylococcal protein A, add 10 L of Bis-tris buffer solution with a pH=7.2 and a concentration of 20 mM to suspend the cell, Then use a high-pressure homogenizer to crush twice at a pressure of 800 bar, and collect about 10 L of supernatant by centrifugation. The resulting solution was added with hydrochloric acid to adjust the pH to 3.0, centrifuged, and the supernatant was adjusted to pH = 7.2 with sodium hydroxide, thereby obtaining a protein solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption performance | aaaaa | aaaaa |
| Adsorption performance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com