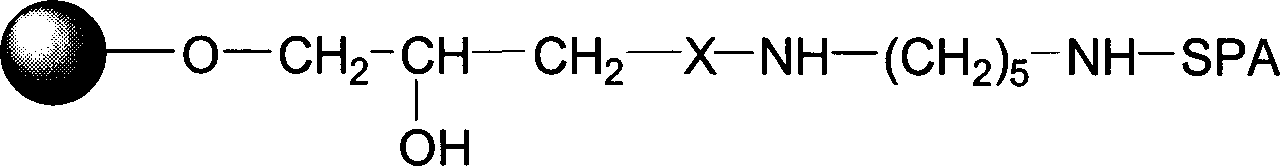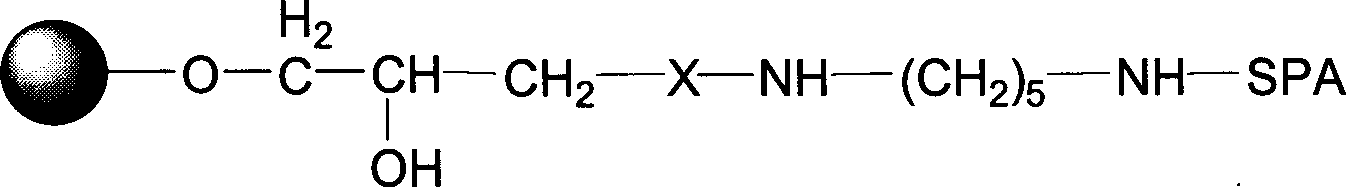Protein A immunoadsorption material for eliminating pathogenic antibody and its complexes, and synthesizing method and application thereof
An immunoadsorbent material and a complex technology, which is applied to protein A immunoadsorbent materials for removing pathogenic antibodies and their complexes and their synthesis and application fields, can solve the problem that the spacer arm of the immunoadsorbent material is not long enough and the ammonia water reactivity is low. , low antibody adsorption efficiency, etc., to achieve the effect of improving clinical treatment effect, improving binding efficiency, and good regeneration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
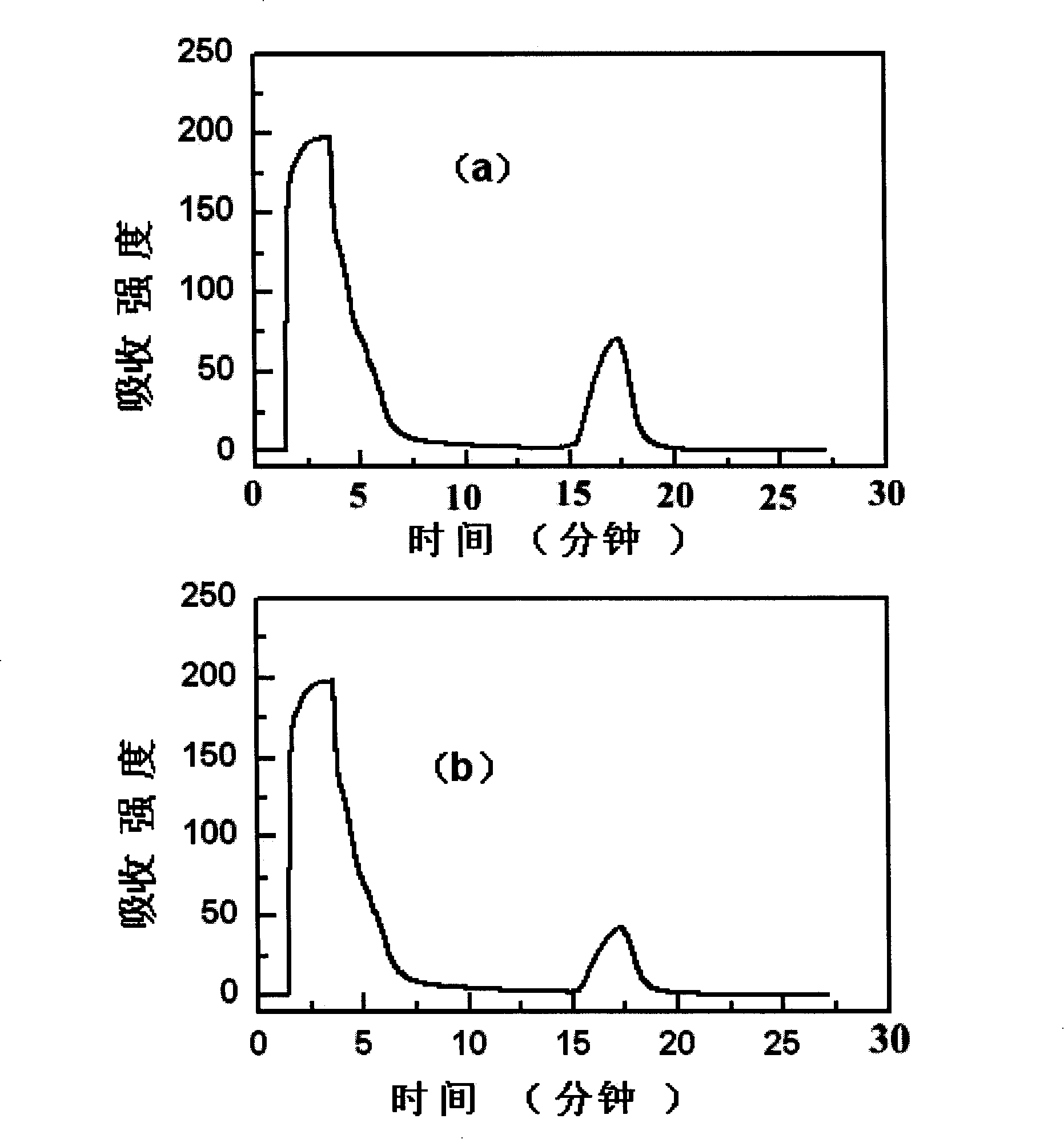
Image
Examples
Embodiment 1
[0042] Synthesis of Active Sepharose 6FF Agarose Gel Containing Epoxy Groups
[0043] Add 1 liter of trade name Sepharose 6FF agarose gel, 1500 ml of 2.5 mol / L NaOH aqueous solution, 3 grams of sodium borohydride into a 5 L reactor, mix well, add about 300 mL of epibromohydrin, and place In a constant temperature shaker, react at 30°C for 4 hours. After the reaction was completed, the gel was filtered and rinsed with a large amount of distilled water until neutral. Store the activated medium at 4°C for later use. The number of epoxy groups in the gel activated by this method was detected by the sodium thiosulfate method, and it was measured that there were at least 50 μmol of epoxy active groups per milliliter.
Embodiment 2
[0045] Synthesis of active Sepharose 6FF agarose gel containing amino groups
[0046] 1. Reaction of epoxy-active Sepharose 6FF agarose gel with diethylenetriamine
[0047] Add 1 liter of epoxy-active Sepharose 6FF agarose gel synthesized in Example 1, 1.5 L of 0.1 mol / L borate buffer solution, and control the pH value in the range of 7.0 to 9.0. Ethylenetriamine was reacted at a constant temperature of 50°C for 2 hours. After the reaction stopped, rinse with 1mol / L sodium chloride solution and disinfected water in large quantities to remove residual diethylenetriamine to obtain an activated agarose gel containing amino groups.
[0048] 2. Reaction of epoxy-active Sepharose 6FF agarose gel with ethylenediamine
[0049] In the 5L reactor, add 1 liter of epoxy-active Sepharose 6FF agarose gel synthesized in "Example 1", 1.5L of borate buffer solution of 0.1mol / L, and the pH value is controlled in the scope of 7.0~9.0, Add 160 mL of ethylenediamine, and react at a constant tem...
Embodiment 3
[0053] Synthesis of Active Sepharose 6FF Agarose Gel Containing Aldehyde Group
[0054] Add 1 liter of aminoactive Sepharose 6FF agarose gel synthesized in "embodiment two" (1, 2 or 3) in the reactor of 5L, 0.1mol / L borate buffer solution 1.2mL, control system pH value at 8 or so, add 200mL glutaraldehyde, and react at a constant temperature of 30°C for 3 hours. After the reaction was stopped, the residual glutaraldehyde was washed away with a large amount of water to obtain an agarose gel containing aldehyde groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com