Monoclonal antibody, hybridoma, immunoassay method and diagnosis kit
A monoclonal antibody, diagnostic kit technology, applied in the direction of anti-enzyme immunoglobulin, anti-bacterial immunoglobulin, immunoglobulin, etc., can solve the diagnosis of antibody titer and specificity changes, poor specificity, polyclonal antibody Tests are difficult to control quality and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] [Monoclonal antibody preparation]
[0113] (1) Preparation of Helicobacter pylori coccal cell suspension (immunogen)
[0114] Helicobacter pylori (ATCC 43504) was streak cultured step by step on a brain-heart drip agar medium (product of Difco) supplemented with 5% defibrinated horse blood. Plates were incubated at 37°C for 3-4 days in a microaerobic environment, followed by 7 days at 37°C in anaerobic environment, resulting in spherical cells. Each colony was scraped off with a platinum ring or the like, and suspended in phosphate-buffered saline (PBS). Centrifuge at 10,000xg at 4°C for 10 minutes to collect Helicobacter pylori, suspend in 0.5% formaldehyde, and keep the suspension at 4°C for 4 days to inactivate it. Then, the cells were washed by resuspension in PBS, followed by centrifugation at 10,000 xg for 10 minutes at 4°C, and then resuspension in PBS in triplicate.
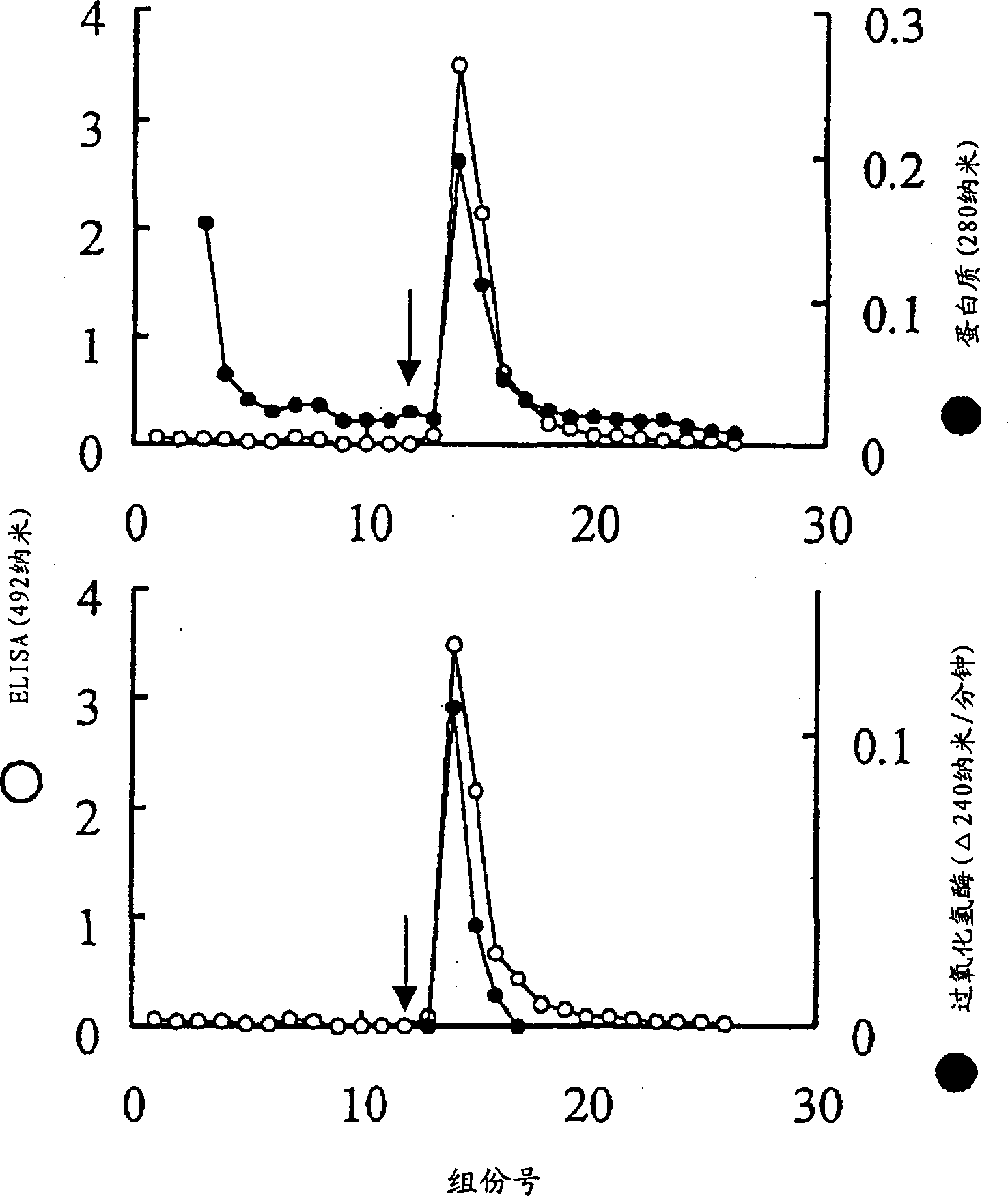
[0115] (2) Preparation of Helicobacter pylori spherical cell breakdown product (immunogen) ...
Embodiment 2
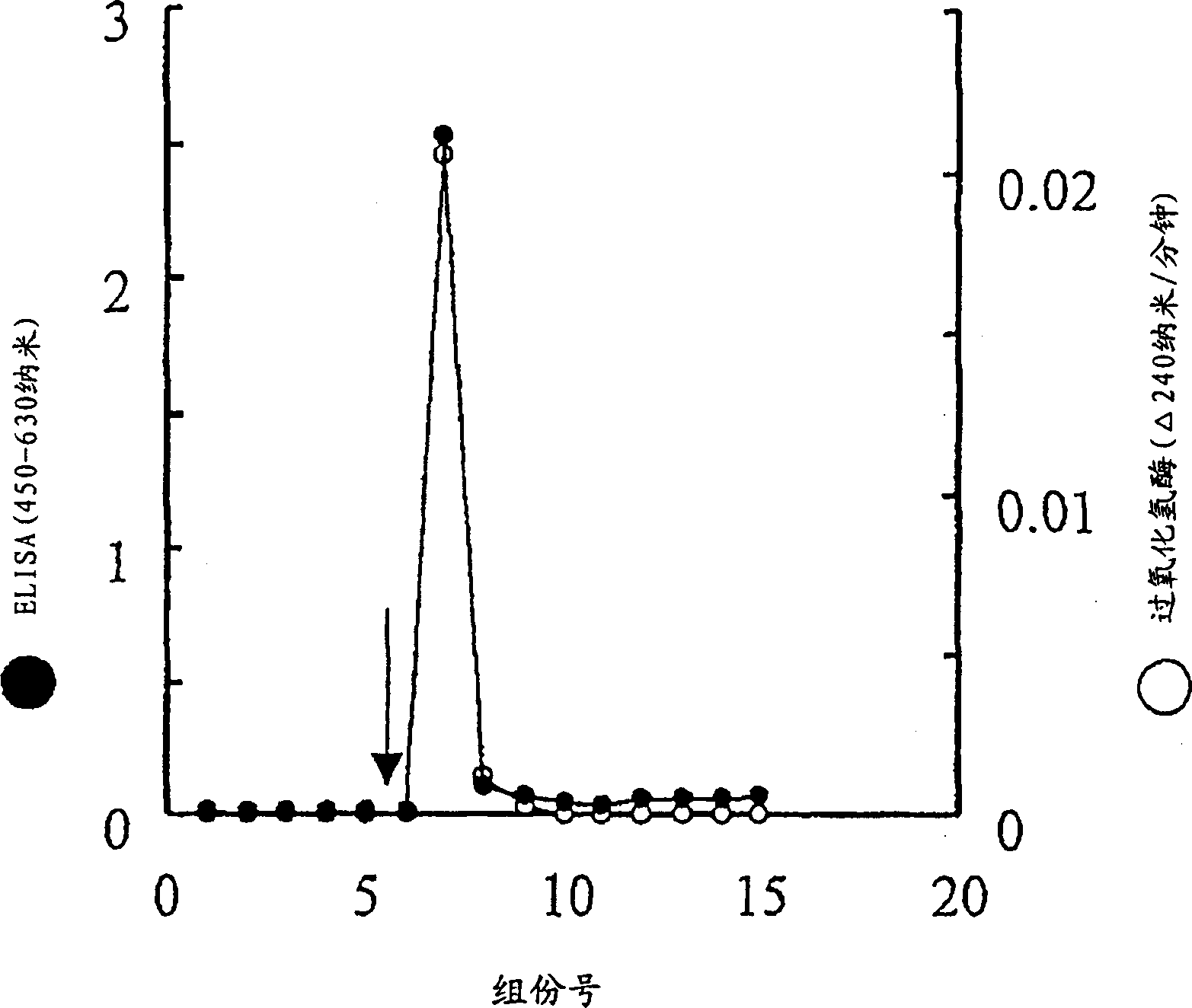
[0122] [Using sandwich ELISA to select monoclonal antibodies that specifically recognize Helicobacter pylori in gastrointestinal excreta]
[0123] (1) Preparation of monoclonal antibody immobilized plate
[0124] The ascitic fluid of 32 clones was diluted twice with PBS, 1 ml each, 2 ml of saturated ammonium sulfate was added dropwise, and the mixture was left at 4°C for 4 hours. The mixture was then centrifuged at 3000 rpm for 20 minutes, the pellet was suspended in 2 ml of PBS and dialyzed. These monoclonal antibodies were immobilized in 96-well ELISA plates in the following manner. That is, each monoclonal antibody was diluted to 5 µg / ml, and 0.2 ml of the dilution was added to each well of a 96-well plate. After overnight at 4°C, the plate was washed with PBS, and then 0.25 ml of 1% skim milk-PBS was added to each well, and the plate was allowed to stand at 4°C for 1 hour for blocking. Then, the plate was washed with the above washing solution.
[0125] (2) Preparation...
Embodiment 3
[0132] [Comparison of reactivity among strains and reactivity with other types of bacteria]
[0133] (1) Preparation of Helicobacter pylori cell suspension
[0134] Various Helicobacter pylori strains (ATCC 43504; Tokai University Hospital clinical isolate No. 130 and 112; Hyogo Medical College clinical isolate Nos. 526, 4484, 5017, 5025, 5049, 5142, 5287, 5308, 5314 and 5330). Plates were cultured at 37°C in a microaerobic environment for 3-4 days to obtain spiral cell colonies, or cultured at 37°C in anaerobic environment for 7 days, resulting in spherical cells. Each colony was scraped off with a platinum ring or the like, and suspended in PBS. Cells were collected by centrifugation at 10,000xg at 4°C for 10 minutes, suspended in 0.5% formaldehyde, and kept at 4°C for 4 days to inactivate. Then, the cells were washed by resuspension in PBS, followed by centrifugation at 10,000 xg for 10 minutes at 4°C, and then resuspension in PBS in triplicate. Helicobacter pylori heli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com