Primer composition, kit and method for detecting mutation of exon 19 of human EGFR gene
A primer composition and exon technology are applied in the field of biotechnology and medicine, can solve the problems of high tumor cell content, cannot be widely promoted, and have multiple tumor tissues, and achieve good reliability, good human-machine interface, The effect of a high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
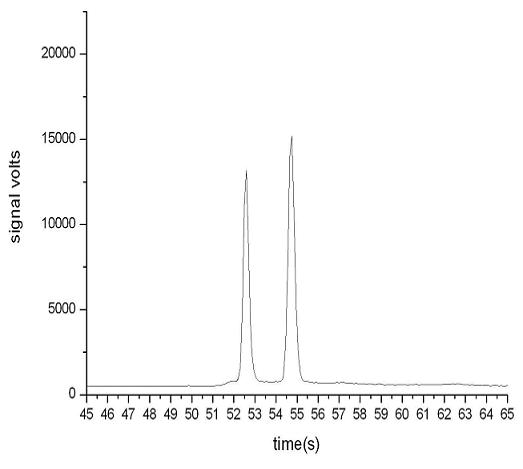
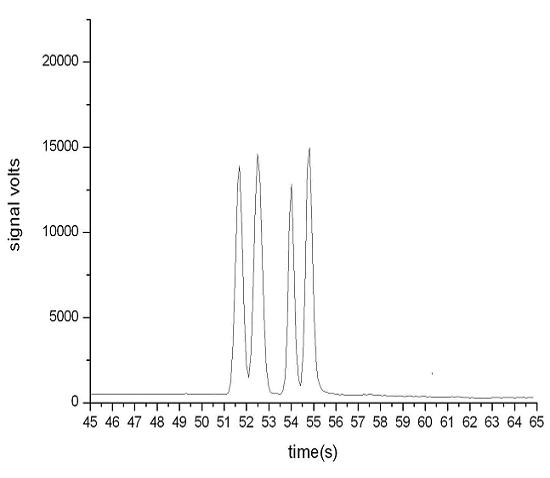
[0032] Example 1 Determination of EGFR mutation sites to be detected
[0033] According to the report on the study of EGFR mutation in NSCLC patients (source: Dong Qianggang, Han Baohui, Study on gene mutation of EGFR exon 19 in serum cell-free DNA of advanced lung cancer, Chinese Journal of Tumor, 2006, 1: 59-61), it was found that EGFR gene 19 The common mutation types of exon 1 genes are shown in Table 1.
[0034] Table 1 Mutation types of exon 19 of EGFR gene
[0035] mutation type
Nucleic acid sequence (5'-3') 2416-2448
Amino acid sequence (744-754)
Wild type
ATCAAGGAATTAAGAGAAGCAACATCTCCGAAA
LKELREATSPK
Deletion E746-A750 (1)
ATCAA----------------ACATCTCCGAAA
LK-----TSPK
Deletion E746-A750 (2)
ATCAAG---------------ACATCTCCGAAA
LK-----TSPK
Deletion L747-E749insP
ATCAAGGA----------GCAACATCTCCGAAA
LKE---ATSPK
Deletion L747-S752
ATCAAGGA-------------------CCGAAA
LKE------PK
D...
Embodiment 2
[0038] Example 2: The composition and preparation of the kit
[0039] The kit of the present invention is composed of a PCR reaction solution, a mixture of primer combination solutions, a negative control, a positive control and sterilized double distilled water.
[0040] 1. Prepare PCR reaction solution: Tris-HCl (pH 8.4) 40 mM, MgCl 2 15~24mM, KCl 50mM, (NH 4 ) 2 SO 4 10 mM, dNTP 0.5 mM, and the final concentration of Taq DNA polymerase was 0.06-0.10 U / μL. where MgCl 2 The optimal concentration is 20 mM, and the optimal final concentration of Taq DNA polymerase is 0.08 U / μL.
[0041] 2. Prepare the mixed solution of the primer composition: each 10 μM of the primers shown in SEQ ID No:1 and SEQ ID No:2, and the primer shown in 20 μM of SEQ ID No:3 are mixed and prepared, and SEQ ID No: The volume ratio of the primer shown in 1, the primer shown in SEQ ID No:2 and the primer shown in SEQ ID No:3 is 1:1:1;
[0042] 3. Sterilized double distilled water (ddH 2 O);
...
Embodiment 3
[0051] Example 3 : Extraction of DNA from clinical samples
[0052] The scope of application of clinical samples includes surgically resected fresh pathological tissues, paraffin-embedded case tissues, whole blood, plasma, serum, pleural effusion, etc. The DNA extraction kit uses the blood / tissue genome extraction reagent produced by Ningbo Ji Nei Biotechnology Co., Ltd. Kit (registration number: Zheyong Food and Drug Administration (Quasi) Zi 2010 No. 1400013), operate according to the kit instructions. In this example, this kit was used to extract genomic DNA from 1 normal physiological tissue sample and 9 fresh pathological tissue samples of clinical non-small cell lung cancer. Other pathological tissue DNA samples were numbered 2-10 in sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com