Rapid detection kit for nuclear matrix protein 22 and application thereof
A nuclear matrix protein and detection kit technology, applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of inability to meet clinical requirements, poor stability of chemiluminescent immunoassay kits, etc., and achieve the benefit of early Diagnosis and prognostic treatment, improvement of sensitivity and accuracy, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
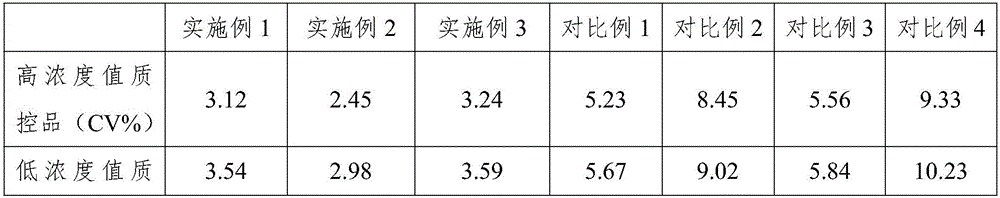
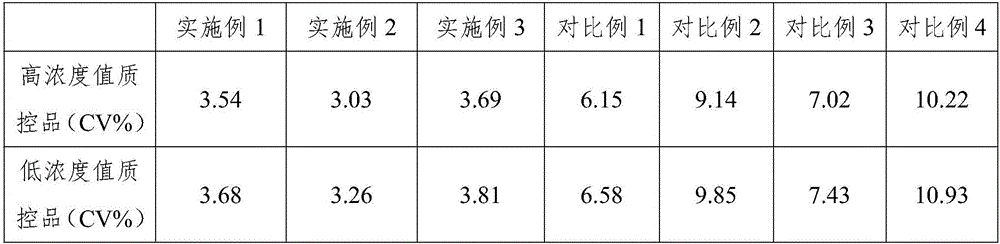
Embodiment 1
[0035] Embodiment 1, a kind of rapid detection kit of nuclear matrix protein 22
[0036] The preparation of the rapid detection kit of nuclear matrix protein 22
[0037] 1. Preparation of solid-phase carrier coated with nuclear matrix protein 22 monoclonal antibody:
[0038] The nuclear matrix protein 22 monoclonal antibody was added into a sodium citrate buffer with a pH of 4.2 to make a coating solution. The added amount of the nuclear matrix protein 22 monoclonal antibody was 0.8% of the volume of the sodium citrate buffer. Add the above coating solution into a 96-well microwell plate, 100 μL per well, place at 4°C overnight, shake off the coating solution, then add 300 μL of blocking solution to each well, place at room temperature for 3-5 hours, shake off the blocking solution, and dehumidify at room temperature Dry for 24 hours to obtain;
[0039] The blocking solution is composed of the following components: sodium citrate buffer 30g / L, tetradecyltrimethylammonium chl...
Embodiment 2
[0047] Embodiment 2, a kind of rapid detection kit of nuclear matrix protein 22
[0048] The preparation of the rapid detection kit of nuclear matrix protein 22
[0049] 1. Preparation of solid-phase carrier coated with nuclear matrix protein 22 monoclonal antibody:
[0050] The nuclear matrix protein 22 monoclonal antibody was added into a sodium citrate buffer solution with a pH of 4.2 to make a coating solution, and the addition amount of the nuclear matrix protein 22 monoclonal antibody was 1.0% of the volume of the sodium citrate buffer solution. Add the above coating solution into a 96-well microwell plate, 100 μL per well, place at 4°C overnight, shake off the coating solution, then add 300 μL of blocking solution to each well, place at room temperature for 3-5 hours, shake off the blocking solution, and dehumidify at room temperature Dry for 24 hours to obtain;
[0051] The blocking solution consists of the following components:
[0052] Sodium citrate buffer 45g / L,...
Embodiment 3
[0060] Embodiment 3, a kind of rapid detection kit of nuclear matrix protein 22
[0061] The preparation of the rapid detection kit of nuclear matrix protein 22
[0062] 1. Preparation of solid-phase carrier coated with nuclear matrix protein 22 monoclonal antibody:
[0063] The nuclear matrix protein 22 monoclonal antibody was added into a sodium citrate buffer solution with a pH of 4.2 to make a coating solution, and the addition amount of the nuclear matrix protein 22 monoclonal antibody was 1.2% of the volume of the sodium citrate buffer solution. Add the above coating solution into a 96-well microwell plate, 100 μL per well, place at 4°C overnight, shake off the coating solution, then add 300 μL of blocking solution to each well, place at room temperature for 3-5 hours, shake off the blocking solution, and dehumidify at room temperature Dry for 24 hours to obtain;
[0064] The blocking solution consists of the following components:
[0065] Sodium citrate buffer 50g / L,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com