Mercury ion detection reagent and detection method
A technology for detecting reagents and mercury ions, applied in Raman scattering, material excitation analysis, etc., can solve problems such as laborious, incapable of direct detection, and difficult to meet environmental monitoring requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
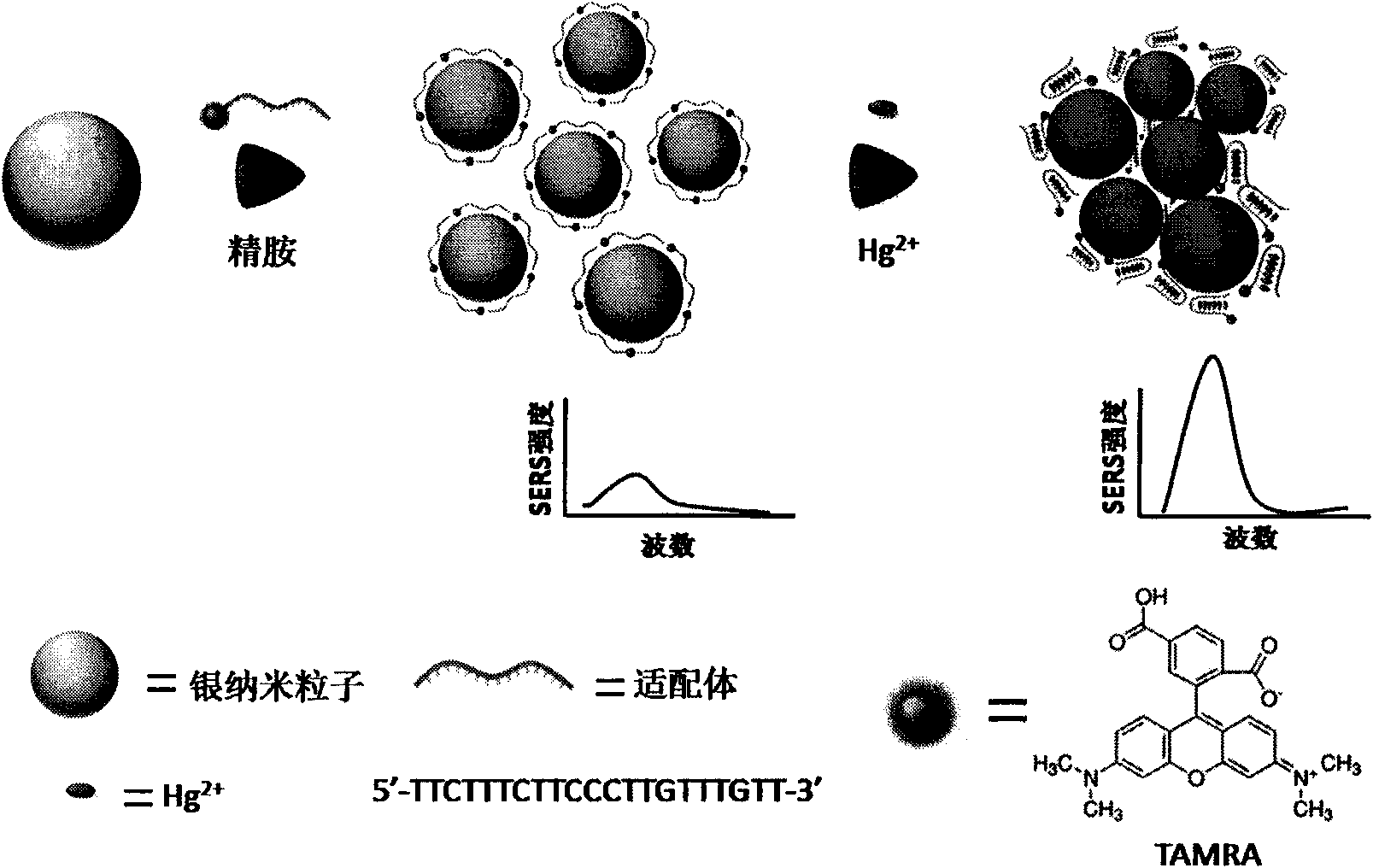
[0031] a. Synthesis of silver nano-sol at room temperature (N.Leopold and B.Lendl.J.Phys.Chem.B., 2003,107:5723-5727): first add 5mL hydrochloric acid to a conical flask containing 84mL deionized water Hydroxyl ammonia (0.03mol / L) and 1mL NaOH (0.6mol / L) with vigorous stirring. Then add AgNO at a constant speed of 2-3 drops / second 3 (0.01mol / L) a total of 10mL. After continuing to stir for 1 hour, bright yellow AgNPs with a particle size of 35±8nm were obtained, which were used as the stock solution nanoparticle sol for future use.
[0032] b. Add 1 μL of aptamer solution (100 μmol / L) to 200 μL of fresh AgNPs and mix well. The sequence of the aptamer is: 5'-TAMRA-TTCTT TCTTCCCTTG TTTGTT-3'.
[0033] c. Add 5 μL spermine (100 μmol / L) to the above solution, and mix well (D.Graham, W.E.Smith, A.M.T.Linacre, C.H.Munro, N.D.Watson and P.C.White.Anal.Chem., 1997, 69: 4703- 4707).
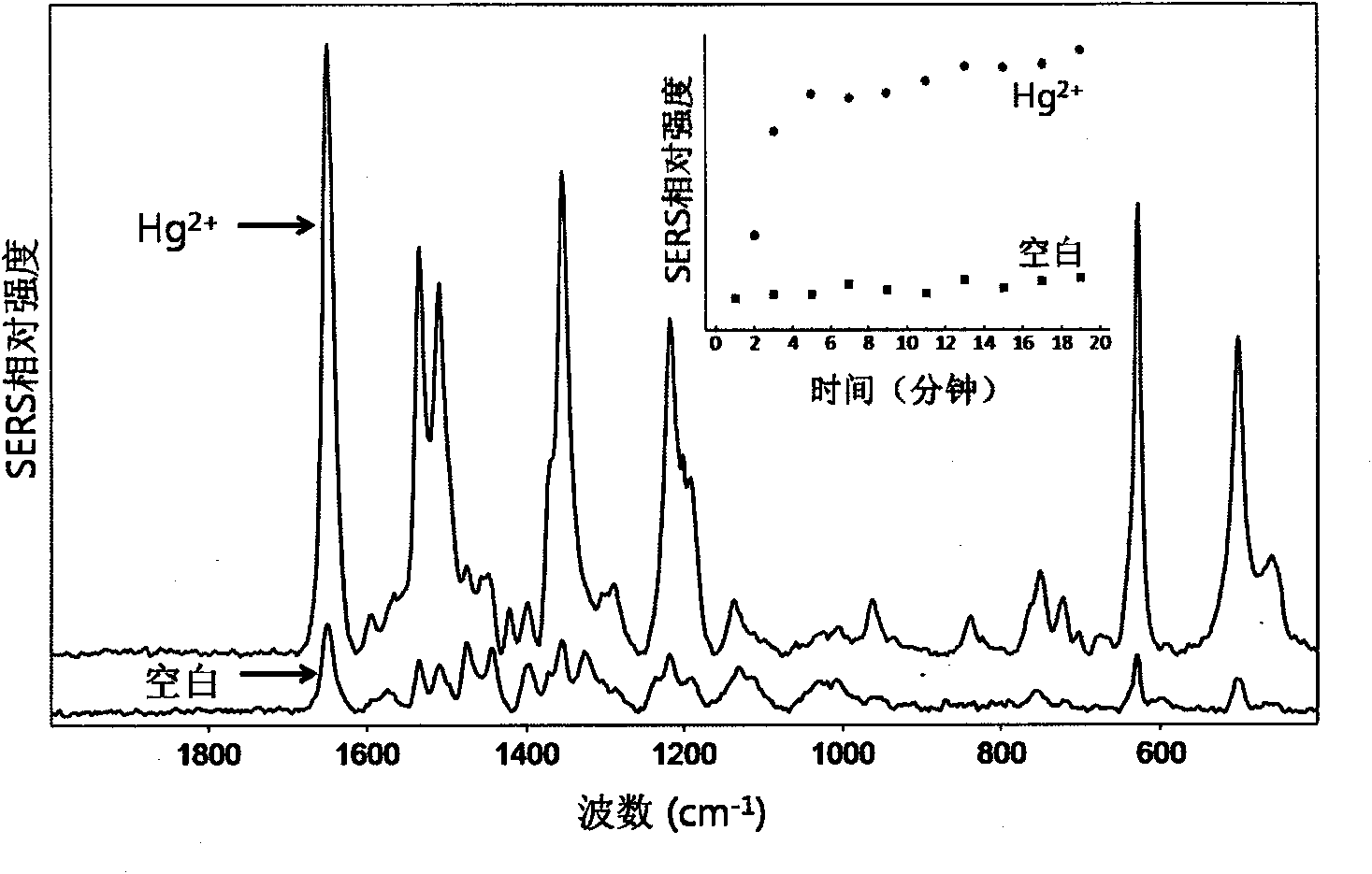
[0034] d. Add a certain concentration of Hg to 90 μL sol respectively 2+ Solution 10μL, incubate...
Embodiment 2
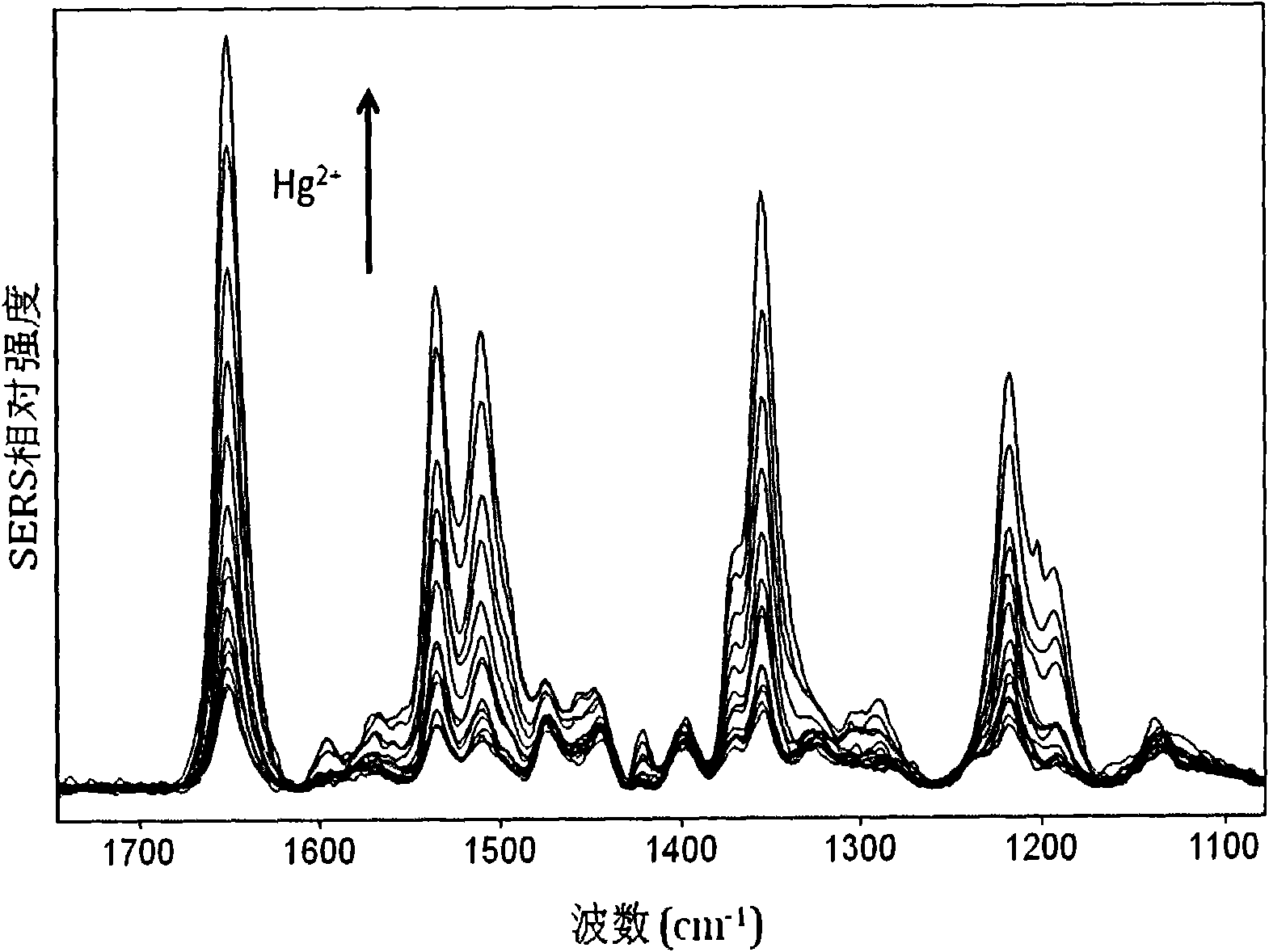
[0040] During the implementation process, the silver sol synthesis method in Example 1 was used to obtain fresh silver sol, which was centrifuged, and a small amount of water was added to obtain AgNPs whose particle concentration was 2.5 times the initial concentration. Add 2.5 μL aptamer solution (100 μmol / L) to 200 μL fresh AgNPs and mix well. The sequence of the aptamer is the same as in Example 1. Add 10 μL of spermine (100 μmol / L) to the above solution and mix well. Add high concentration Hg to the above sol respectively 2+ solution, 1:1 (volume ratio) mixed, incubated for 5min. Use a glass capillary to absorb a little sol and collect Raman scattering signals. The experimental conditions are the same as in Example 1. at 1651cm -1 The intensity of the Raman peak is the standard peak for data processing. The example for Hg 2+ The detection limit is lower than 5nmol / L, for Hg 2+ The linear response range of concentration is 5-150nmol / L, and at the same time, it has h...
Embodiment 3
[0042] a. Same as Example 1; b. Add 1 μL of aptamer solution (100 μmol / L) to 200 μL of fresh AgNPs and mix well. The sequence of the aptamer is: 5'-TAMRA-TTTTTTTTTTTTTTTTTTTTTTTT-3'; c-g are the same as in Example 1. The example for Hg 2+ The detection limit is lower than 2nmol / L, for Hg 2+ The linear response range of concentration is 5-80nmol / L, and at the same time, it has high selectivity, and the whole detection process only takes 5-10 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com