Cell Suspension Preparation Technique and Device
a cell suspension and preparation technique technology, applied in the field of cell suspension preparation technique and device, can solve the problems of requiring considerable skill, time and expensive equipment to perform, and limiting the area able to be covered, so as to reduce the complexity, reduce the complexity, and improve the effect of grafting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Recipient Site
[0116]To optimize the success of the skin graft, the wound was cleaned and assessed to be of the appropriate depth. Further, blood haemostasis was established and the wound checked for evidence of surrounding cellulitis or infection. Techniques for preparing the area included sharp dissection, dermabrasion or laser-resurfacing.
Donor Site Biopsy
[0117]The donor site was chosen to appropriately match the recipient site. The donor site was infiltrated with local anaesthetic and adrenaline underneath the skin near the subcutaneous tissue. This allowed the donor site to be firm and aided in the taking a thin split-thickness biopsy. The dimensions of the biopsy were determined by the size of the surface area of the recipient site to be covered. Typically, the biopsy size has an expansion ratio of 1:10-1:80. In this case, a biopsy size of 2 cm×2 cm was taken from the donor site giving an expansion ratio of 1:60.
Cell Resurfacing Using the Rapid Technique
[0118]Tre...
example 2
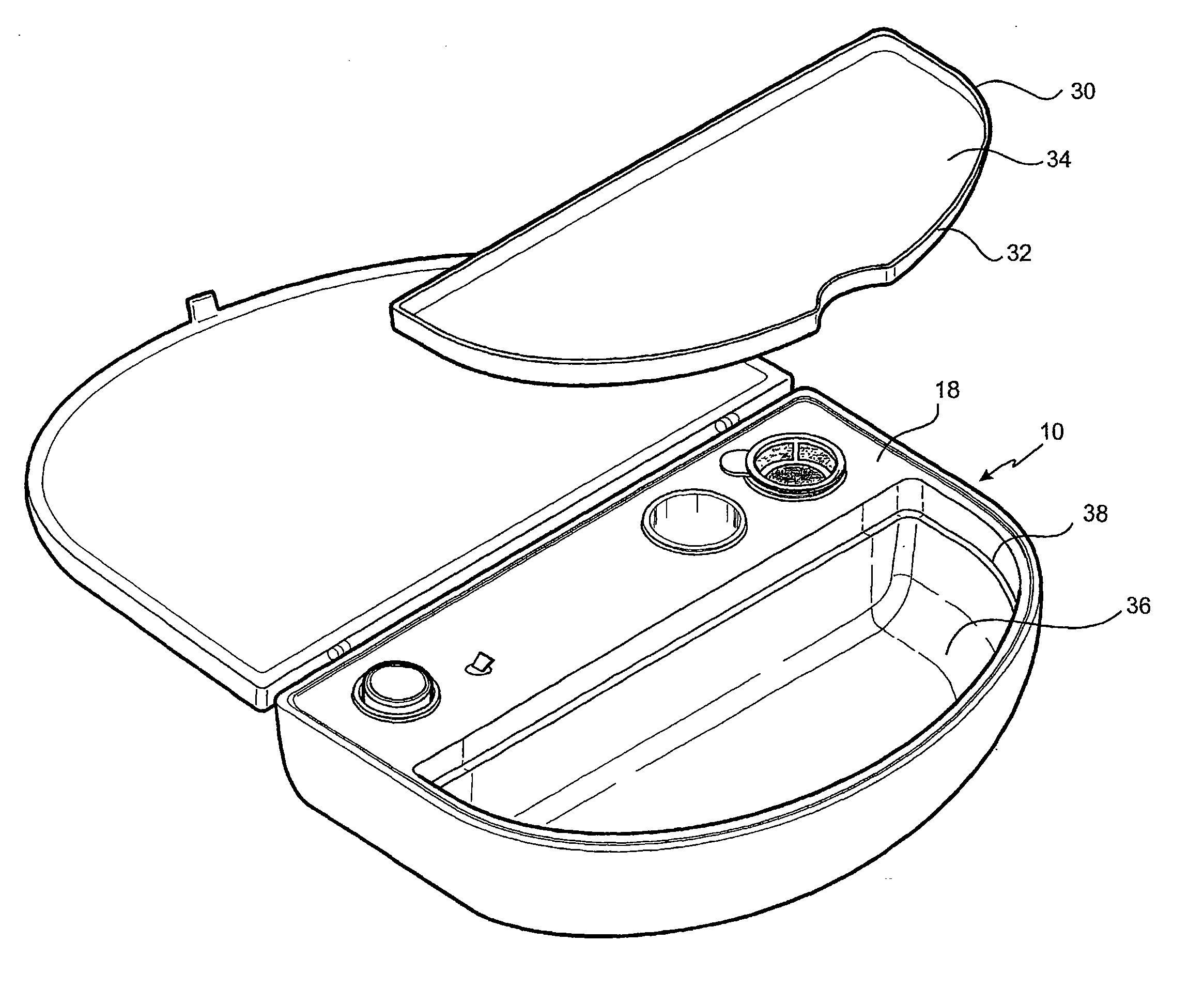
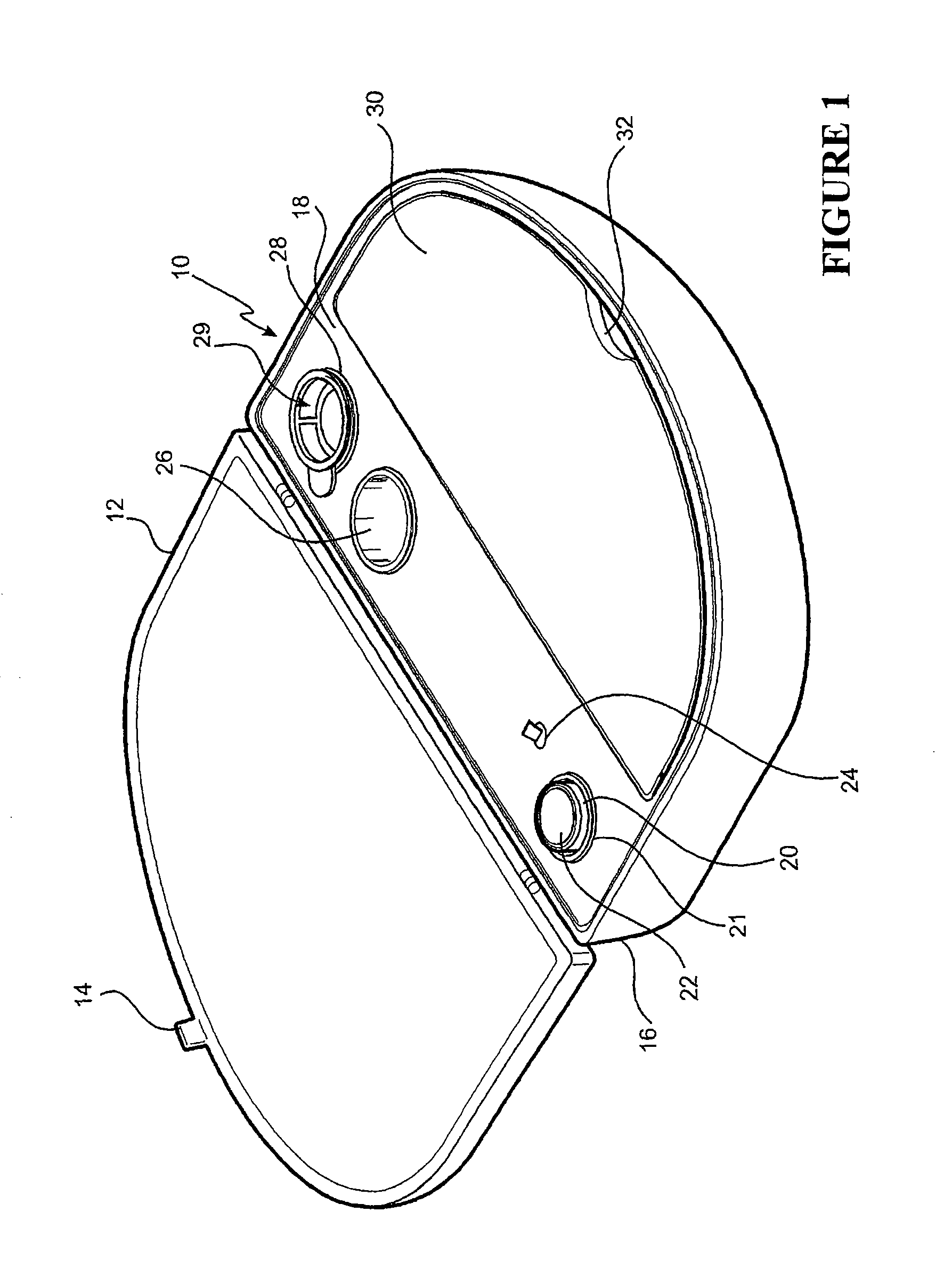
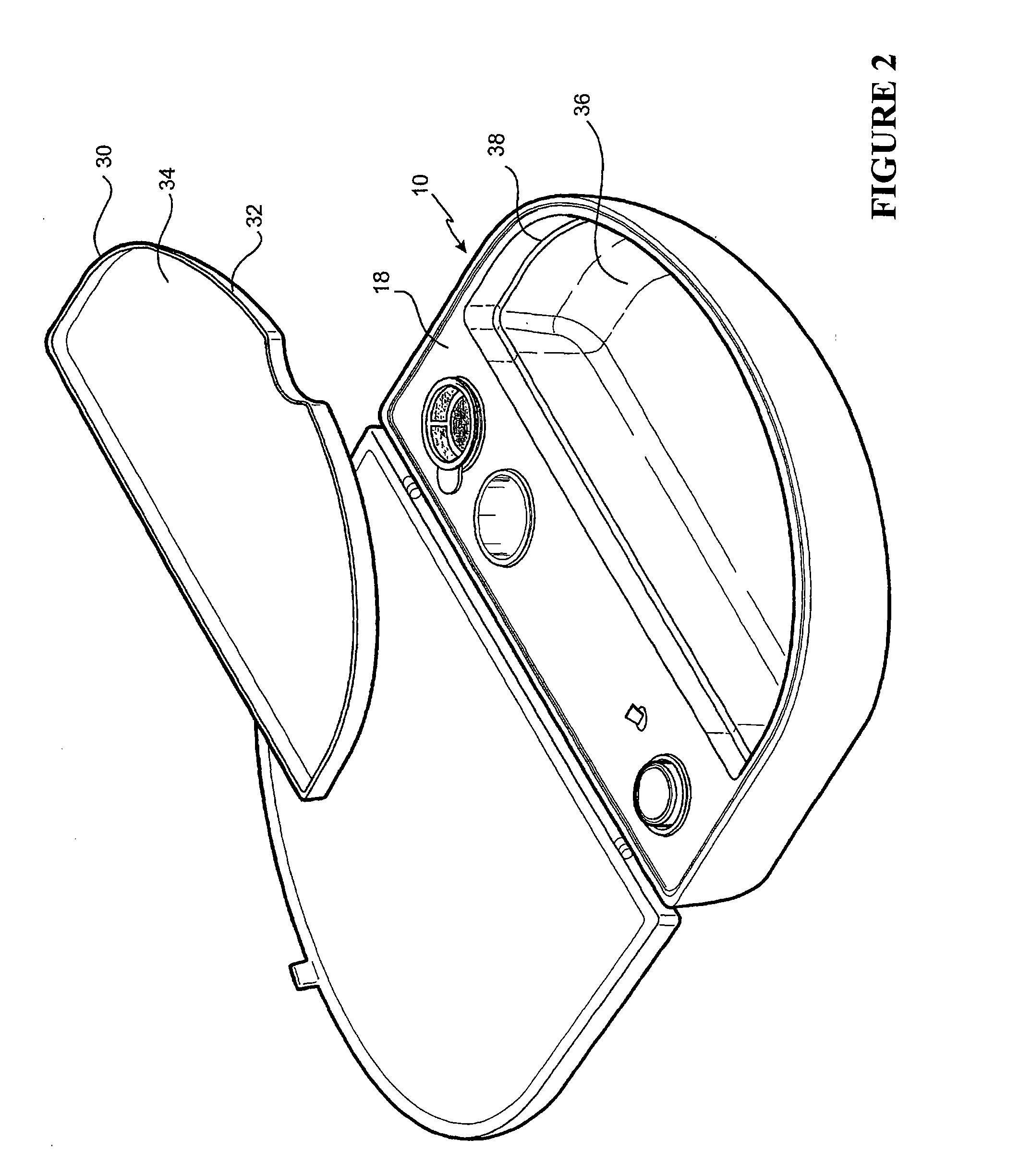
[0128]The embodiment shown in FIG. 1 is directed to a Re-Cell® Rapid Technique cell harvesting apparatus 10 for use in producing a transplantable cellular suspension of living tissue suitable for grafting to a patient.
[0129]As illustrated in FIG. 1 the apparatus includes a closure lid 12 possessing a locking mechanism 14 adapted to releasably engage a base portion 16. The locking mechanism 14 provides a means for closing the apparatus 16 when not in use. Located within the base portion 16 is a first member 18 within which there is provided an aperture 20 in which there is located a vial 22 for the enzyme. Adjacent the aperture there is provided an activation switch 24 capable of activating the heating means (not shown). The first member also provides a fluid containment well 26 and a filter recess 28. As presented in this illustration the filter 29 is shown located in the filter recess. Ordinarily the filter is an optional item included as a separate item in the apparatus.
[0130]The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| suction pressure | aaaaa | aaaaa |
| healing time | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com