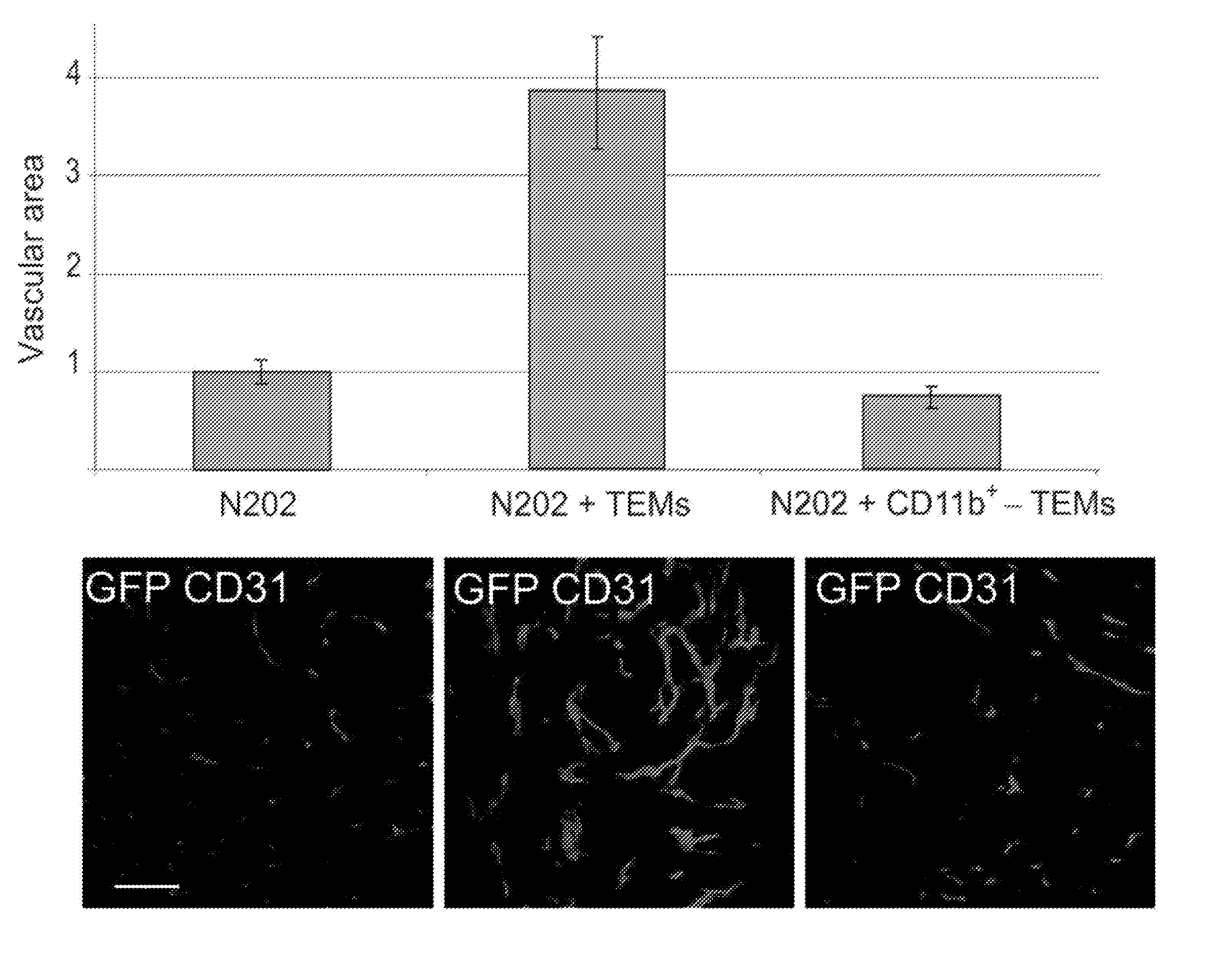Patents
Literature
97 results about "Monocyte production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monocytes are produced by the bone marrow from stem cell precursors called monoblasts. Monocytes circulate in the bloodstream for about one to three days and then typically move into tissues throughout the body. They make up three to eight percent of the leukocytes in the blood.
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS20060029578A1Add supportImprove coagulation/solidificationBiocideOrganic active ingredientsAbnormal tissue growthRepair tissue
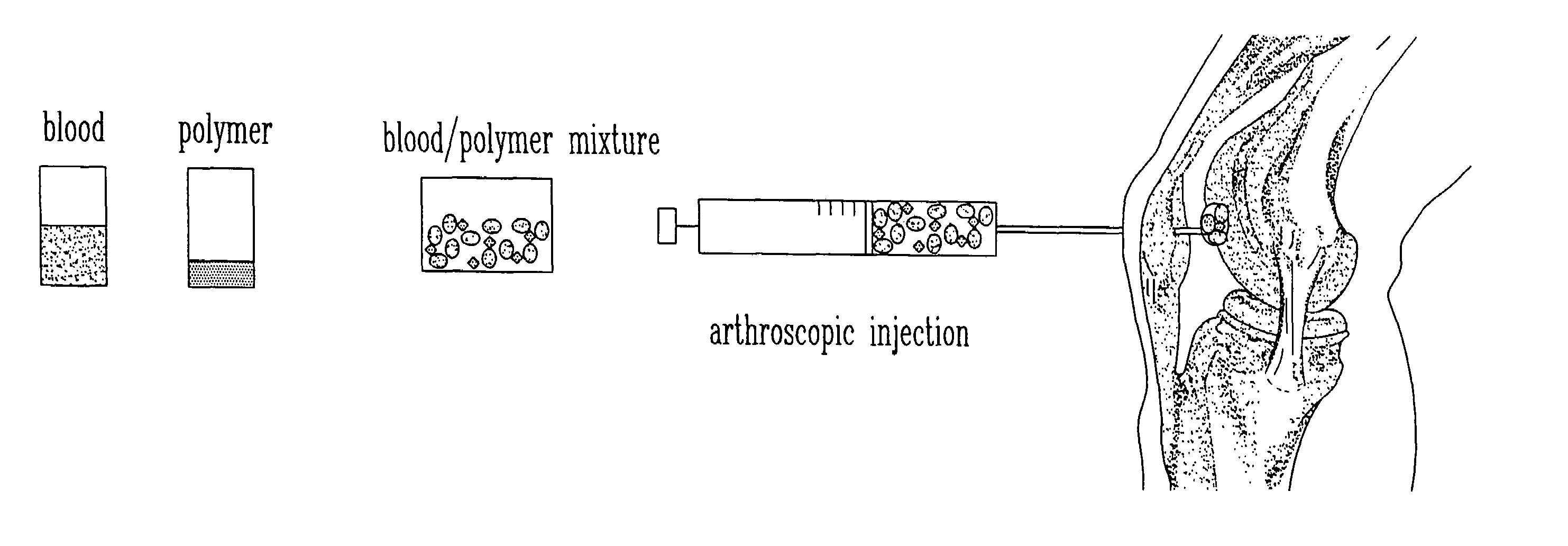
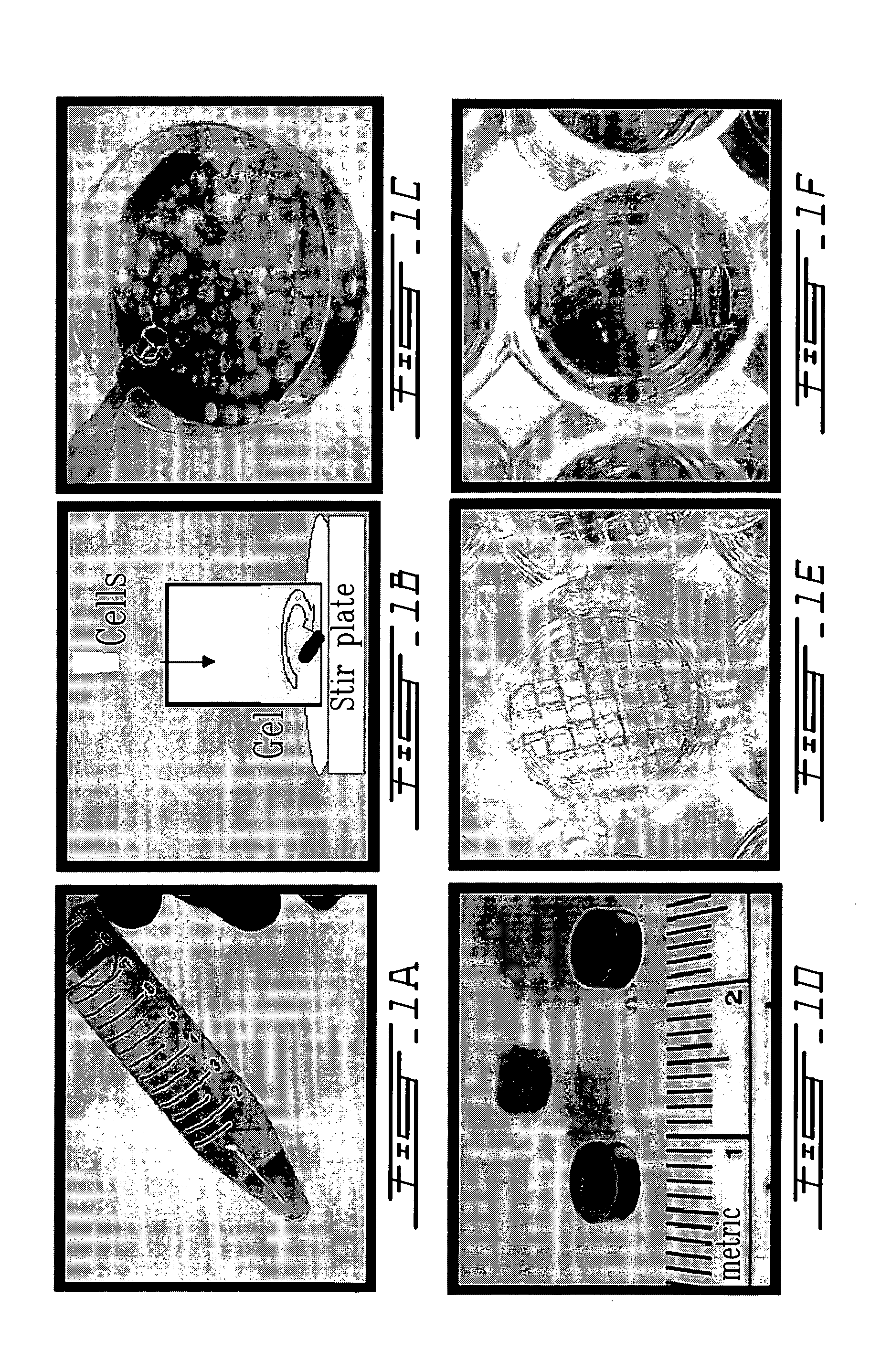
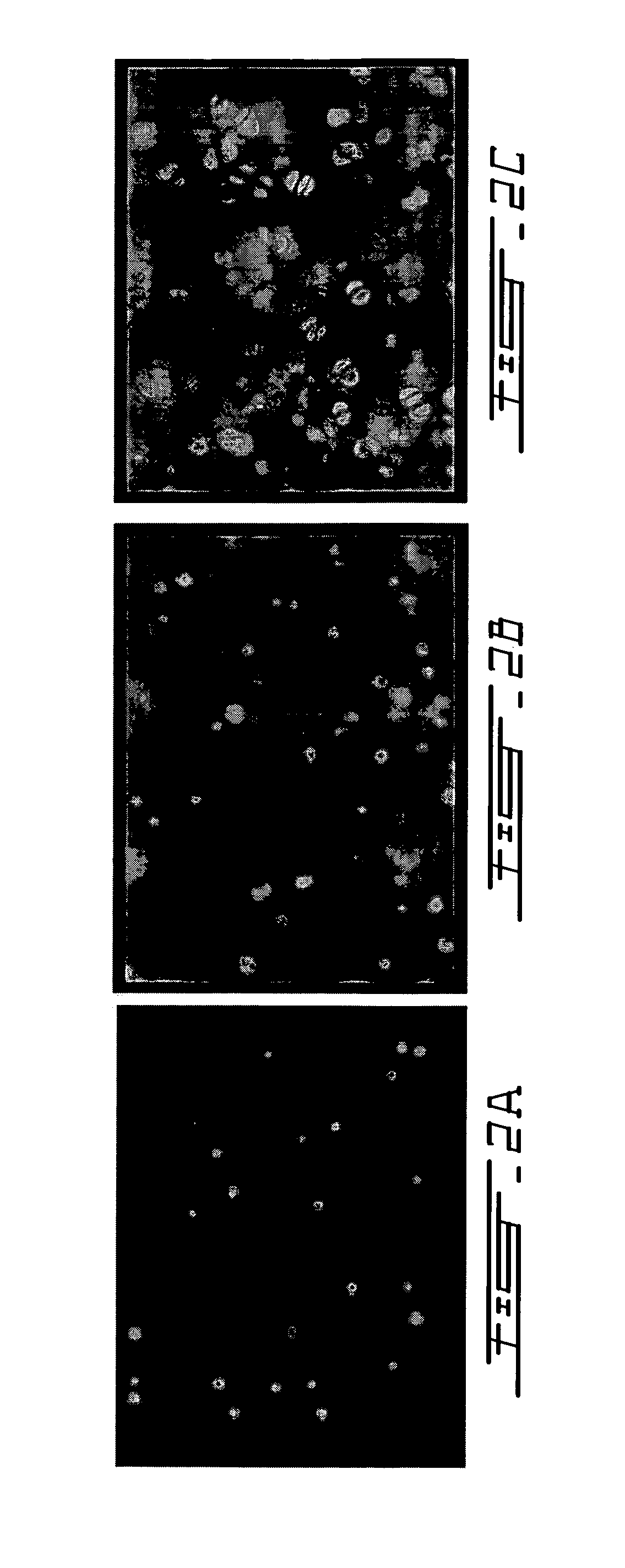
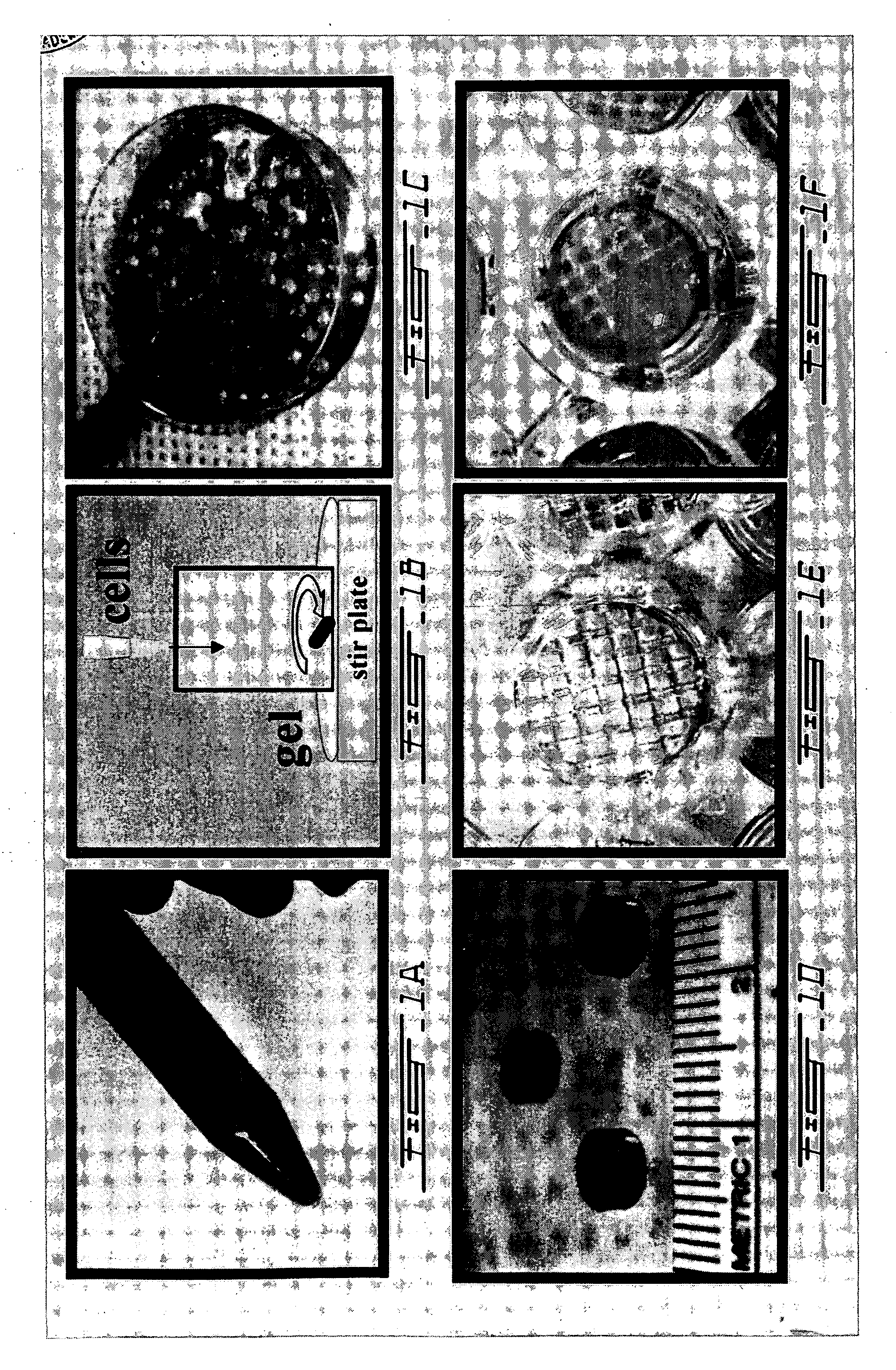
The present invention relates to a new method for repairing human or animal tissues such as cartilage, meniscus, ligament, tendon, bone, skin, cornea, periodontal tissues, abscesses, resected tumors, and ulcers. The method comprises the step of introducing into the tissue a temperature-dependent polymer gel composition such that the composition adhere to the tissue and promote support for cell proliferation for repairing the tissue. Other than a polymer, the composition preferably comprises a blood component such as whole blood, processed blood, venous blood, arterial blood, blood from bone, blood from bone-marrow, bone marrow, umbilical cord blood, placenta blood, erythrocytes, leukocytes, monocytes, platelets, fibrinogen, thrombin and platelet rich plasma. The present invention also relates to a new composition to be used with the method of the present invention.
Owner:SMITH & NEPHEW ORTHOPAEDICS
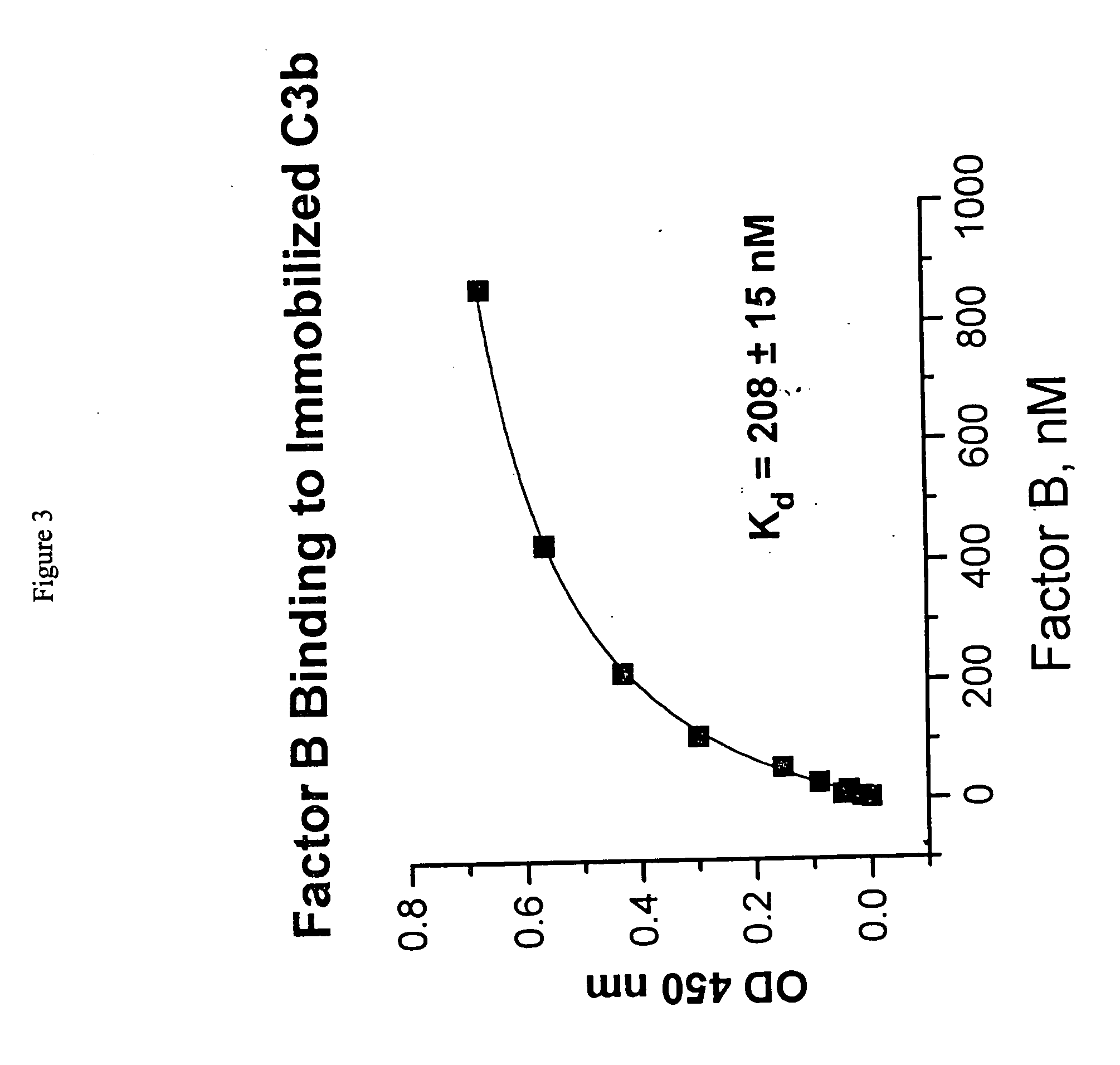
Method of inhibiting factor B-mediated complement activation, and the uses thereof
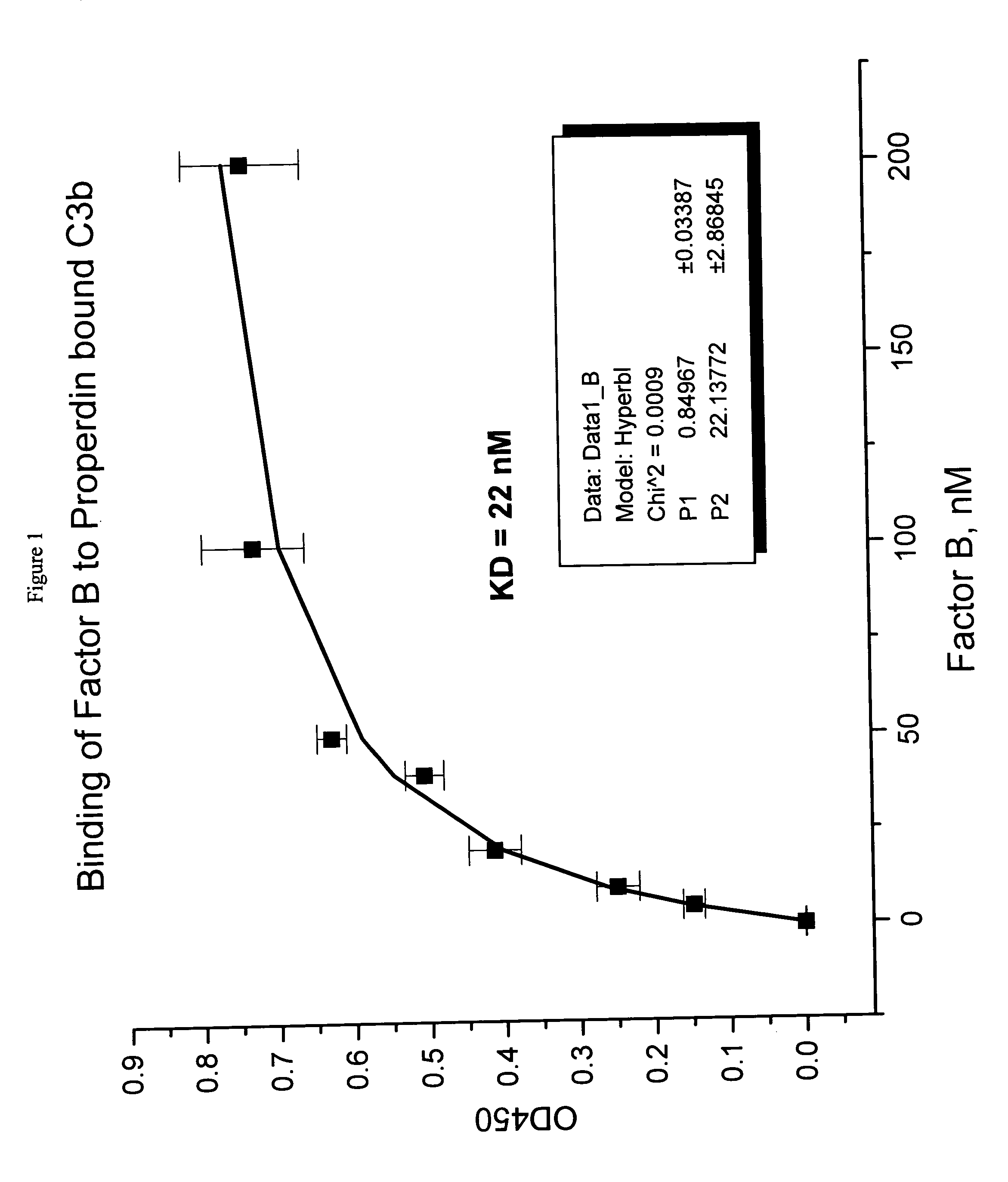
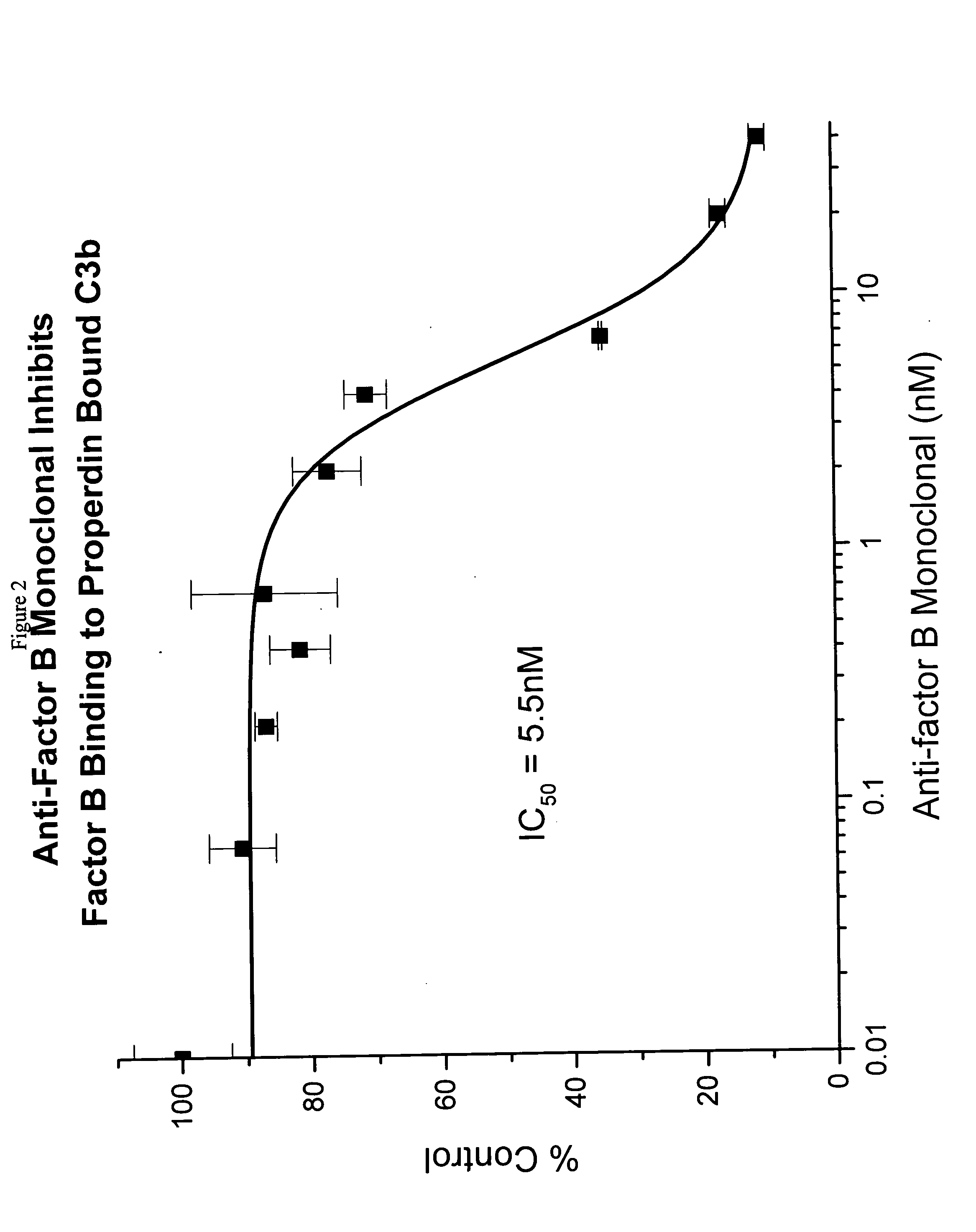
The present invention discloses the method of inhibiting complement activation mediated by factor B inhibitors, that involves: (a) inhibiting factor B binding to properdin-bound C3b; (b) inhibiting the release of Bb; (c) inhibiting the activation of neutrophils, monocytes, platelets, and endothelium; or (d) inhibiting / reducing the formation of PC3bBb, C3a, C5a, and MAC. The present invention also discloses the novel use of factor B inhibitors in the treatment of various immunological disorders, resulting either primarily from direct immune responses such as rheumatoid arthritis, anaphylactic shock, myasthenia gravis, asthma, Alzheimer's disease, and the like, or secondarily from clinical conditions such as cardiopulmonary bypass inflammation, vascular stenosis and restenosis, burn injury, and the like.
Owner:NOVELMED THERAPEUTICS
Tissue transplantation compositions and methods
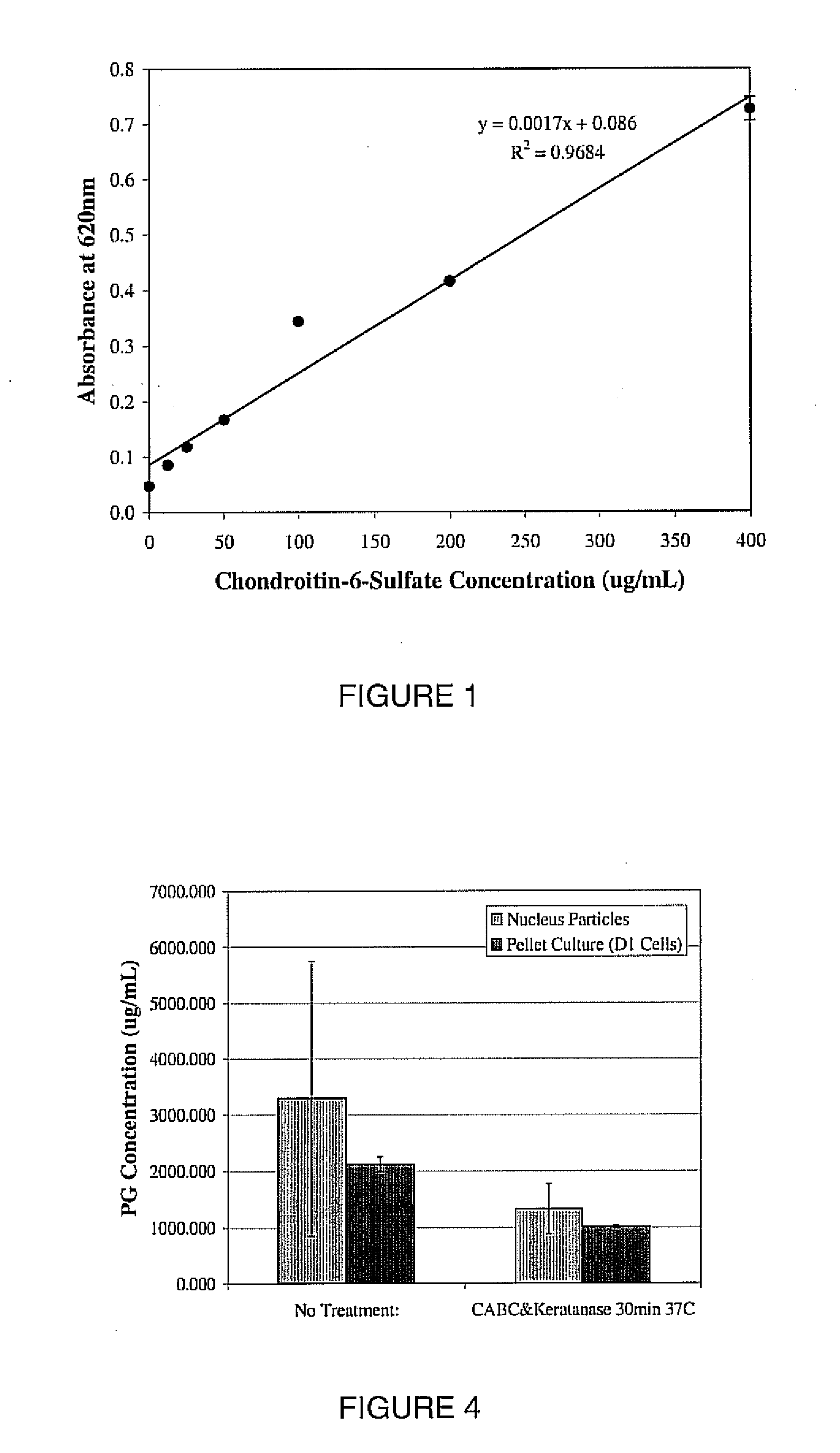
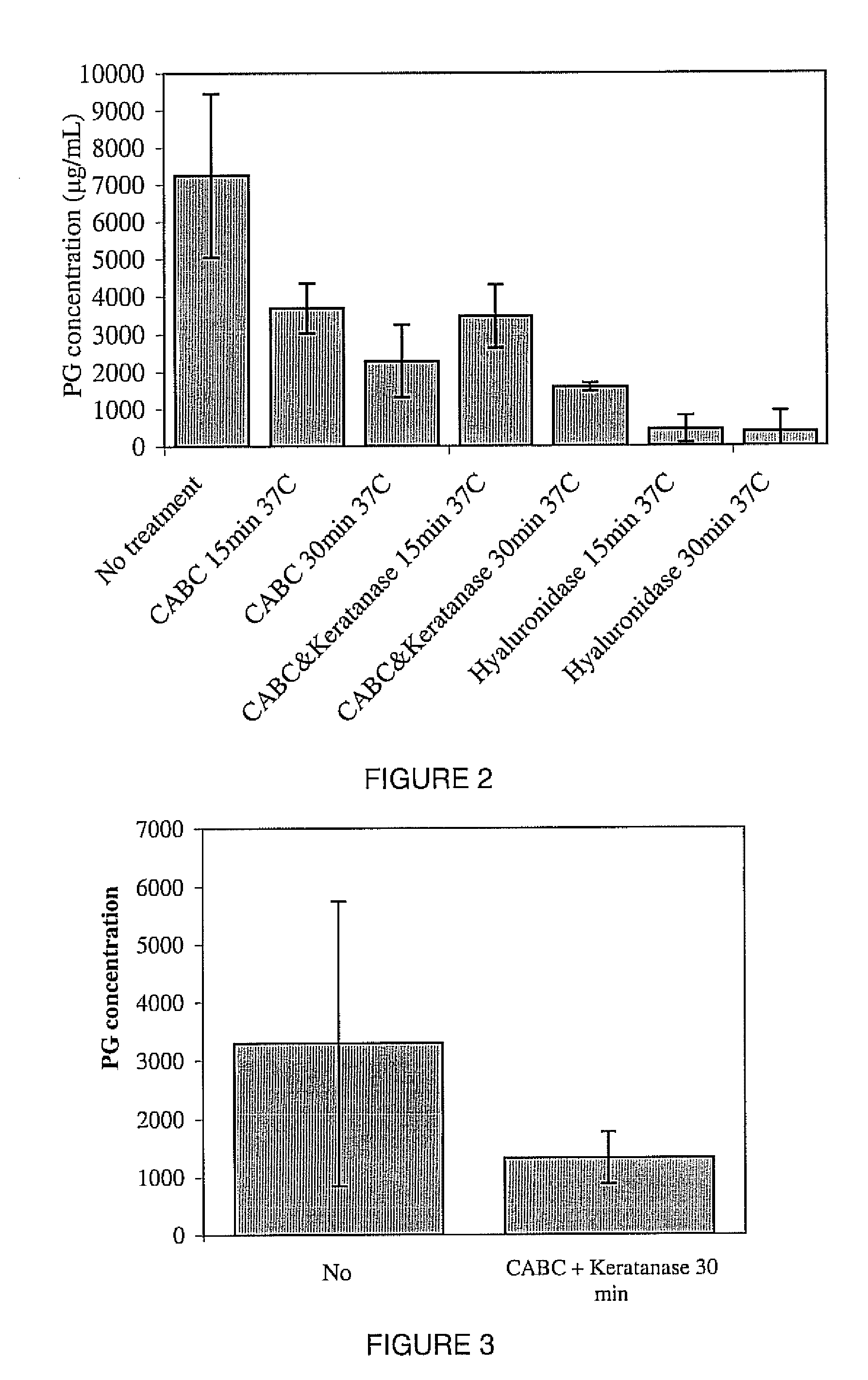
A biomedical material for transplant to a subject is provided according to embodiments of the present invention which includes an isolated donor tissue enzyme-treated to reduce the amount of proteoglycans in the donor tissue compared to untreated tissue. Isolated cells are optionally added to the enzyme-treated donor tissue, including leukocytes, particularly monocytes; macrophages; platelets; cells derived from an intervertebral disc such as chondrocyte-like nucleus pulposus cells; fibrocytes; fibroblasts; mesenchymal stem cells; mesenchymal precursor cells; chondrocytes; or a combination of any of these. The isolated donor tissue is articular cartilage or an intervertebral disc tissue such as nucleus pulposus tissue and / or annulus fibrosis tissue enzyme-treated to remove proteoglycans normally present in these tissues. A biomedical material of the present invention is administered to a subject to treat a disorder or injury, such as a disorder or injury to connective tissue.
Owner:FERREE BRET
Novel receptor trem (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20130150559A1Strong upregulationImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionDc maturation
Novel activating receptors of the lg super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-β. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:NOVO NORDISK AS
Antibodies against csf-1r
ActiveUS20110243947A1Prevent dimerizationInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDocetaxel-PNPDocetaxel
The invention provides a human antibody that binds human CSF-1R with high affinity. Antibodies of the present invention have significant advantages over the antibodies known in the art by being multifunctional: inhibiting signaling of CSF-1R, internalizing and inducing CSF-1R degradation and stimulating ADCC in cell including tumors, macrophages and monocytes. They are also shown to be effective in treating leukemia, breast, endometrial and prostate cancer alone or in combination with docetaxel, paclitaxel, Herceptin® or doxorubicin.
Owner:IMCLONE SYSTEMS
Method of treatment and agents useful for same
InactiveUS7455836B2Reducing level of proliferation and activation and growth and survivalGood effectOrganic active ingredientsBiocideInflammatory mediatorAnimal model
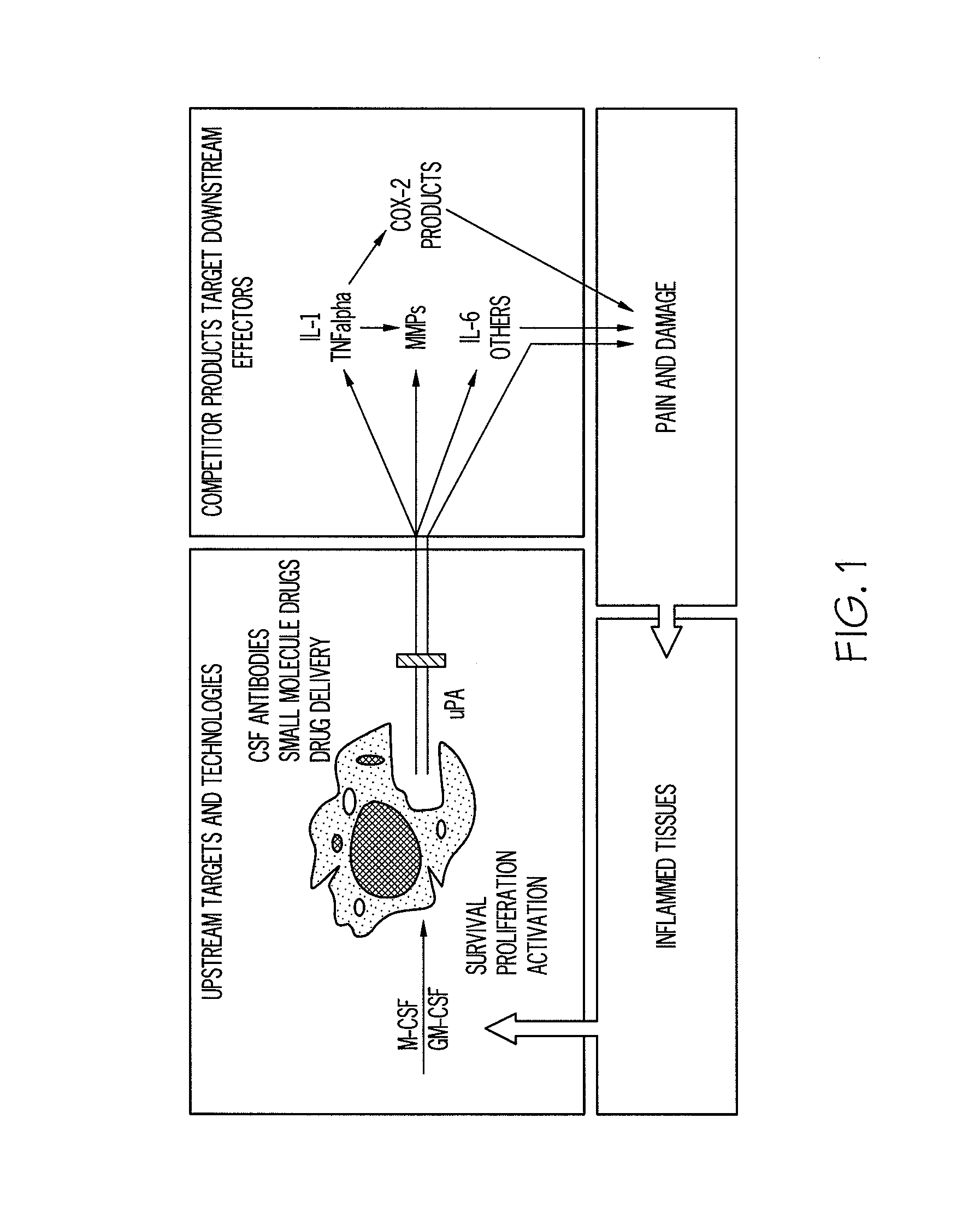
The present invention relates generally to a method for the treatment and prophylaxis of inflammatory conditions. The present invention is predicated in part on the identification of cells of the monocyte / macrophage lineage being critical for inflammation and, in particular, chronic inflammation. In accordance with the present invention, it is proposed that the reduction in levels of monocyte / macrophage-type cells and / or a reduction in the production of inflammatory and pro-inflammatory mediators by these cells, especially locally, is effective in reducing inflammatory conditions. The present invention further provides animal models useful for screening for reducing levels of monocyte / macrophage-type cells and / or reducing the production of inflammatory and pro-inflammatory mediators of these cells.
Owner:MELBOURNE UNIV OF THE +1
Absorbent and Column for Extracorporeal Circulation
InactiveUS20090275874A1Prolong lifeImprove the quality of lifeSemi-permeable membranesHaemofiltrationExtracorporeal circulationZeta potential
The present invention provides an absorbent which can remove cells present in blood including activated leukocytes such as granulocytes and monocytes, and cancer cells as well as can remove cytokines which facilitate the activation of the remaining cells, and further has no concern for pressure loss and has high configuration stability. That is, the present invention provides an absorbent which absorbs the granulocytes and the monocytes in blood, an absorbent for cancer therapy which absorbs an immunosuppressive protein and an absorbent having a bilayer structure of a net and a nonwoven fabric, having a zeta potential of −20 mV or more, as well as a blood circulation column containing any of the absorbents filled therein.
Owner:TORAY IND INC
Purification and uses of dendritic cells and monocytes
InactiveUS7273753B2Enhanced graft acceptanceEnhanced monocyte suppressionArtificial cell constructsFused cellsDiseaseDendritic cell
Methods for the preparation of substantially purified populations of dendritic cells and monocytes from the peripheral blood of a mammal is described. Also described are vaccine compositions and methods for the treatment of certain diseases and medical conditions based on the substantially purified dendritic cells and monocytes.
Owner:CHILDRENS MEDICAL CENT CORP
Method for stimulating wound healing
InactiveUS20050119175A1Promote wound healingAntibacterial agentsBiocideAdditive ingredientFibroblast
The present invention relates to a method for stimulating wound healing, and more particularly to a method for stimulating wound healing in a subject in need thereof, comprising administering to a wound of the subject an effective amount for stimulating wound healing of a composition, wherein the composition comprises p43 having an amino acid sequence set forth in SEQ ID NO: 1 or functional equivalents thereof. The composition used in the method of the present invention can be efficiently utilized for the wound healing, since p43, an effective ingredient of the composition, has an excellent effect on the wound healing by its action including the induction of macrophage / monocyte and endothelial cell, re-epithelization, proliferation of fibroblasts or angiogenesis.
Owner:ATYR PHARM INC
Antagonists of MCP-1 function and methods of use thereof
Compounds which are antagonists of MCP-1 function and are useful in the prevention or treatment of chronic or acute inflammatory or autoimmune diseases, especially those associated with aberrant lymphocyte or monocyte accumulation such as arthritis, asthma, atherosclerosis, diabetic nephropathy, inflammatory bowel disease, Crohn's disease, multiple sclerosis, nephrtitis, pancreatitis, pulmonary fibrosis, psoriasis, restenosis, and transplant rejection; pharmaceutical compositions comprising these compounds; and the use of these compounds and compositions in the prevention or treatment of such diseases.
Owner:TELIK INC +1
Devices and methods for imaging particular cells including eosinophils
InactiveUS20110137178A1Easy to installFaster and high resolutionDiagnostics using spectroscopyDianostics using fluorescence emissionMast cellEosinophil
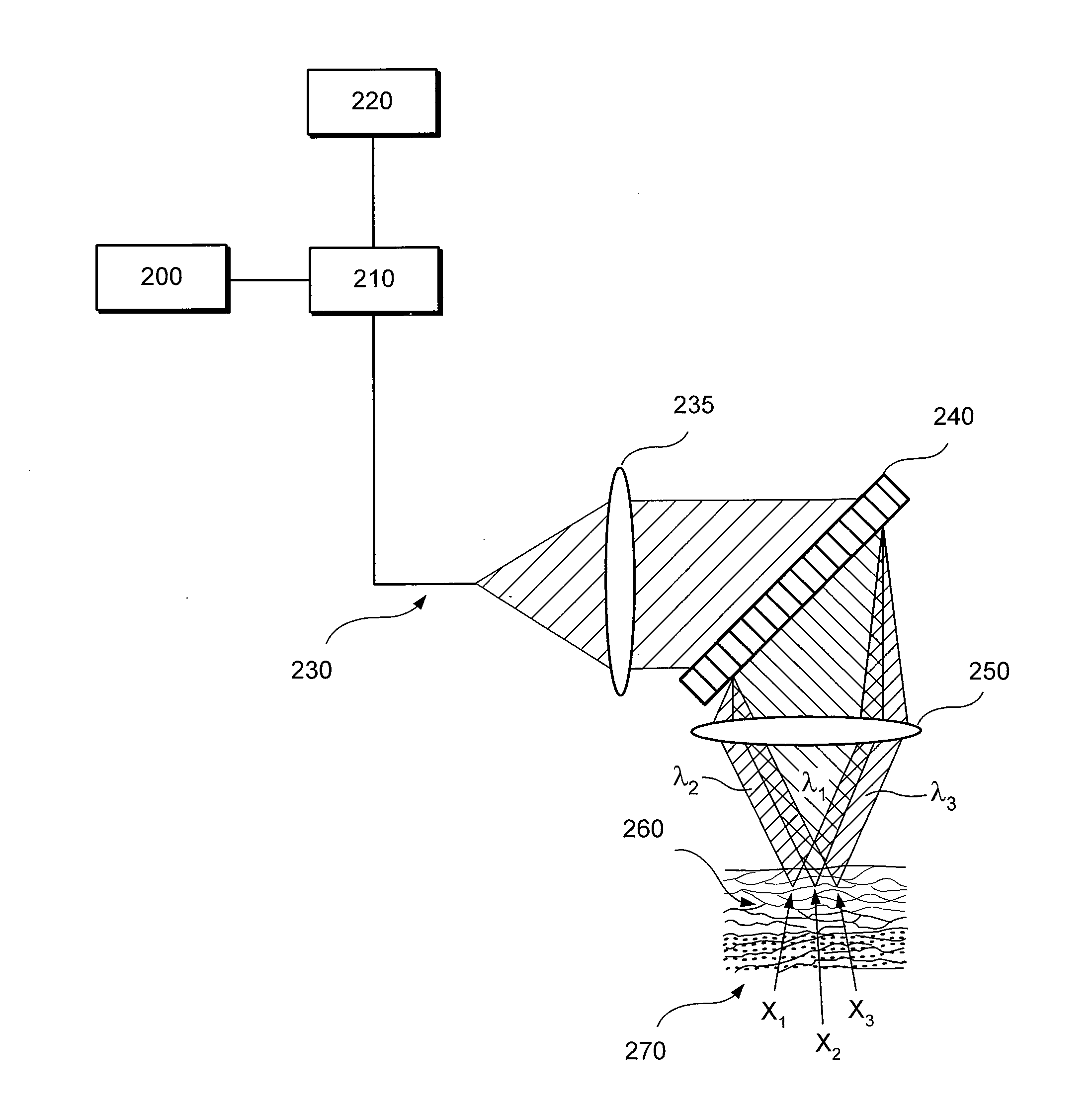
An exemplary embodiment of apparatus and method according to the present disclosure can be provided. For example, using at least one first arrangement, it is possible to direct at least one first electro-magnetic radiation to at least one portion of tissue within a body. Using at least one second arrangement, it is possible to receive at least one second electro-magnetic radiation provided from the portion, which is based on the first electro-magnetic radiation. Further, using at least one third arrangement, it is possible to differentiate at least one particular cell which is eosinophil, mast cell, basophil, monocyte and / or nutrophil from other cells in the portion based on the second electro-magnetic radiation.
Owner:THE GENERAL HOSPITAL CORP
Methods for producing functional antigen presenting dendritic cells using biodegradable microparticles for delivery of antigenic materials
InactiveUS20050158856A1Efficient targetingIncrease probabilityArtificial cell constructsCell culture supports/coatingDendritic cellMicroparticle
Methods are provided for producing functional antigen presenting dendritic cells. The dendritic cells are produced by treating an extracorporeal quantity of a subject's blood to induce differentiation of blood monocytes into dendritic cells. The dendritic cells may be exposed to cellular material encapsulated within a biodegradable polymer material to produce the antigen presenting dendritic cells.
Owner:EDELSON RICHARD L +3
Antagonists of MCP-1 function and methods of use thereof
InactiveUS20050054668A1Useful in treatmentBiocideOrganic chemistryAutoimmune conditionAutoimmune disease
Compounds which are antagonists of MCP-1 function and are useful in the prevention or treatment of chronic or acute inflammatory or autoimmune diseases, especially those associated with aberrant lymphocyte or monocyte accumulation such as arthritis, asthma, atherosclerosis, diabetic nephropathy, inflammatory bowel disease, Crohn's disease, multiple sclerosis, nephrtitis, pancreatitis, pulmonary fibrosis, psoriasis, restenosis, and transplant rejection; pharmaceutical compositions comprising these compounds; and the use of these compounds and compositions in the prevention or treatment of such diseases.
Owner:TELIK INC +1
Monocyte cell
ActiveUS20080057043A1Inhibit angiogenesisSimple procedureBiocideGenetic material ingredientsCD16CD14
Owner:OSPEDALE SAN RAFFAELE SRL +1
Dendritic Cell Compositions and Methods
ActiveUS20090053251A1Great flexibilityIncrease levelAntibacterial agentsSnake antigen ingredientsIncubation periodCD80
Methods are provided for the production of dendritic cells from monocytes that have been incubated at a temperature of 1° C.-34° C. for a period of approximately 6 to 96 hours from the time they are isolated from a subject. After the incubation period, the monocytes can then be induced to differentiate into dendritic cells. Mature dendritic cells made by the methods of the invention have increased levels of one or more of CD80, CD83, CD86, MHC class I molecules, or MHC class II molecules as compared to mature dendritic cells prepared from monocytes that have not been held at 1° C.-34° C. for at least 6 hours from the time they were isolated from a subject. Dendritic cells made by the methods of the invention are useful for the preparation of vaccines and for the stimulation of T cells.
Owner:COIMMUNE INC
Method of detecting cellular immunity and application thereof to drugs
InactiveUS20060035291A1Reduce needAllergen ingredientsCancer antigen ingredientsDiseaseImmune monitoring
It is intended to provide a convenient immunity monitoring system whereby T cell frequencies specific to a plural number of antigen peptides can be assayed by using a relatively small amount of blood. Peripheral monocytes are collected and frequently stimulated with an antigen without directly using any antigen presenting cells. Then T cells specific to the antigen in the thus stimulated peripheral monocytes are detected to thereby detect antigen-specific T cells. thus, diseases such as cancer can be prevented or treated with the use of a peptide having such a function, in particular, cancer tumor-rejection antigen peptide.
Owner:GREEN PEPTIDE CO LTD +1
In vitro production of dendritic cells from CD14+ monocytes
InactiveUS20050008623A1High feasibilityHigh degree of reproducibilityBiocideCosmetic preparationsLangerhan cellMonolayer
The invention relates to the use of CD14+ monocytes for the production of dendritic cells. The invention comprises the use of CD14+ monocytes isolated from peripheral circulating blood for obtaining, by differentiation, at least one mixed population of Langerhans cells and interstitial dendritic cells, both Langerhans cells and interstitial dendritic cells being preconditioned and undifferentiated, and / or differentiated and immature, and / or mature, and / or interdigitated. The invention comprises their use in suspension, monolayer and three-dimensional cell and tissue models. The invention comprises the use of these cells and of these models as study models for the assessment of immunotoxicity / immunotolerance, for the development of cosmetic and pharmaceutical active principles and for the development and implementation of methods of cell and tissue therapy.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
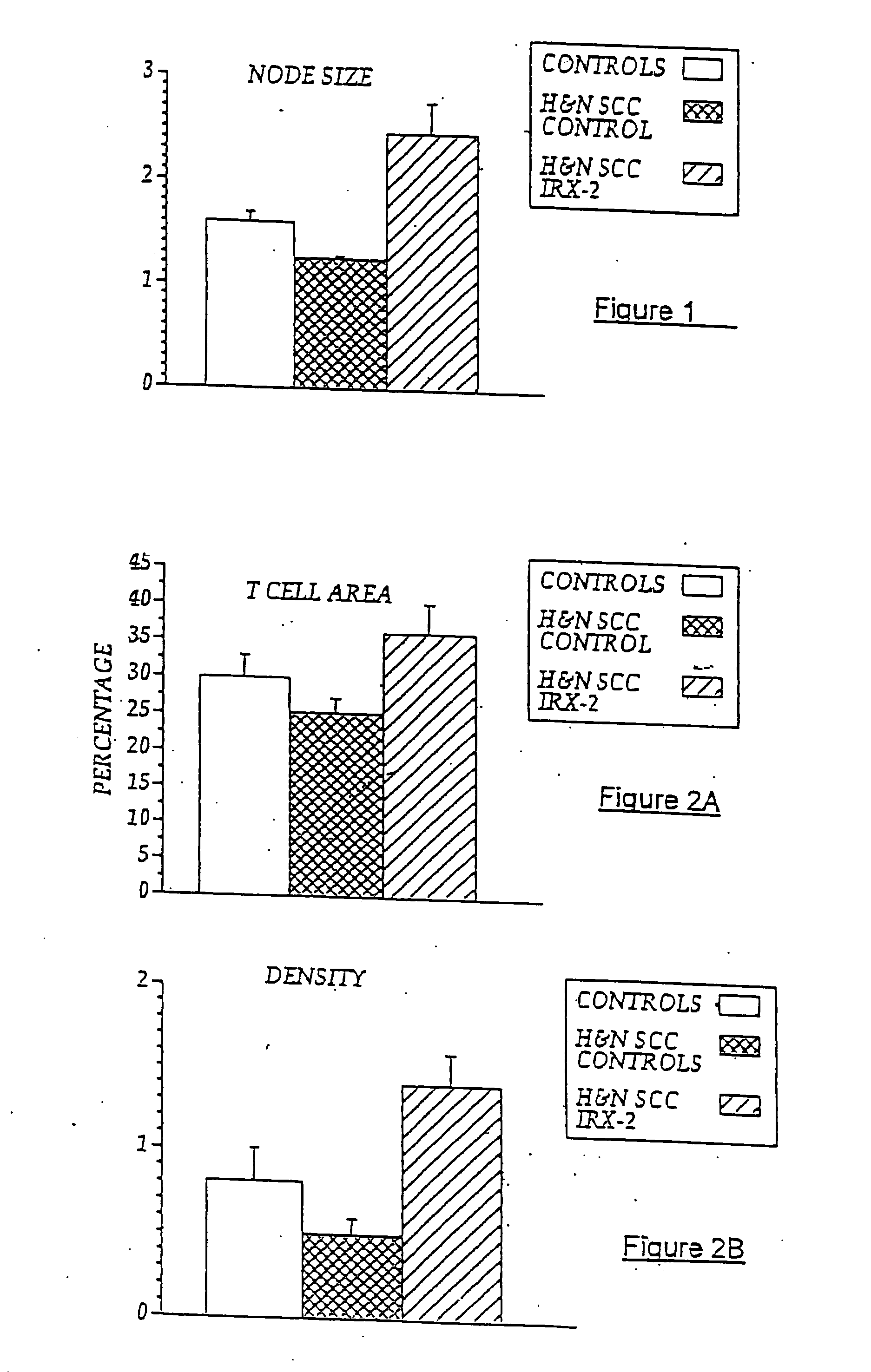
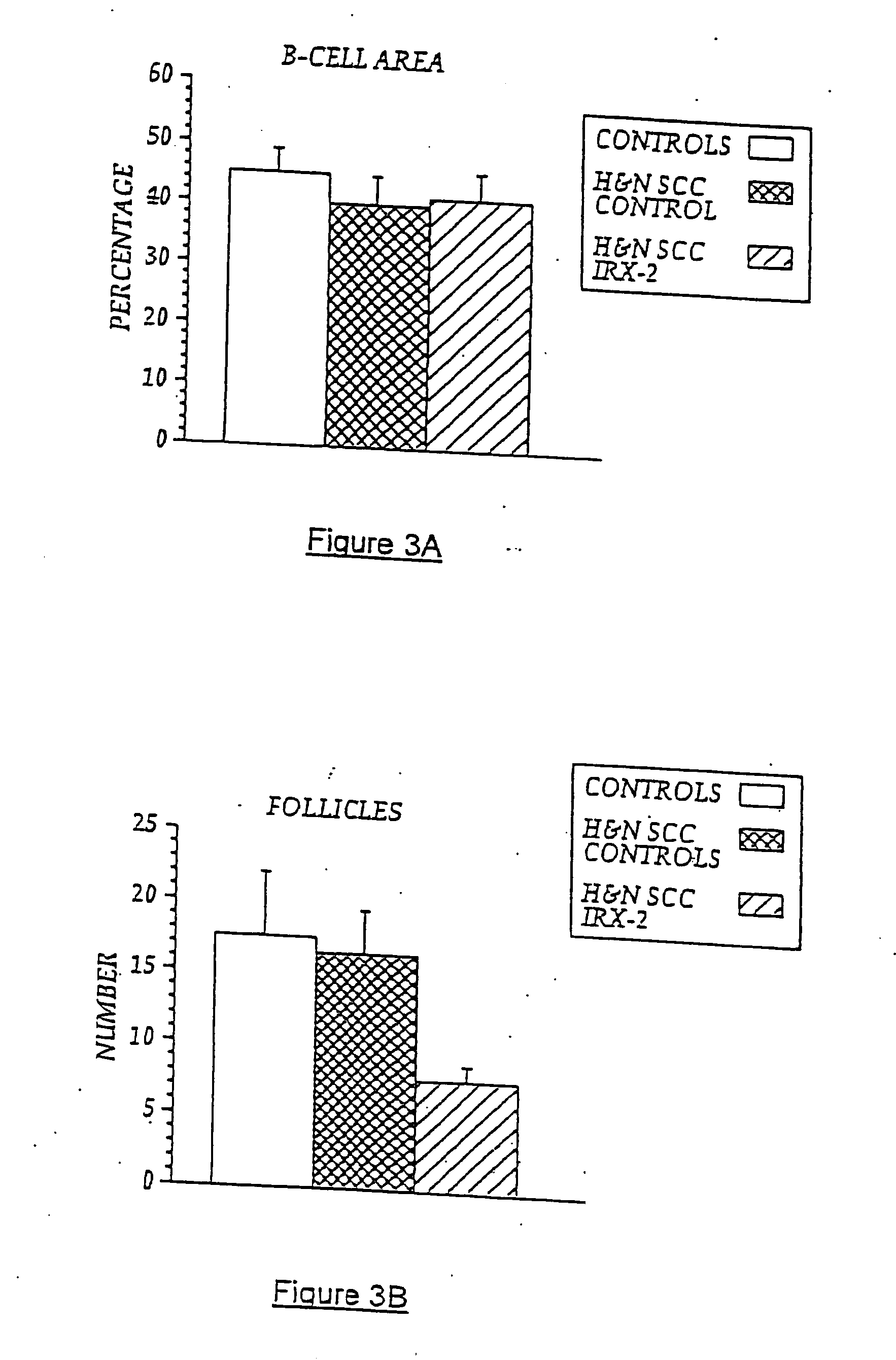
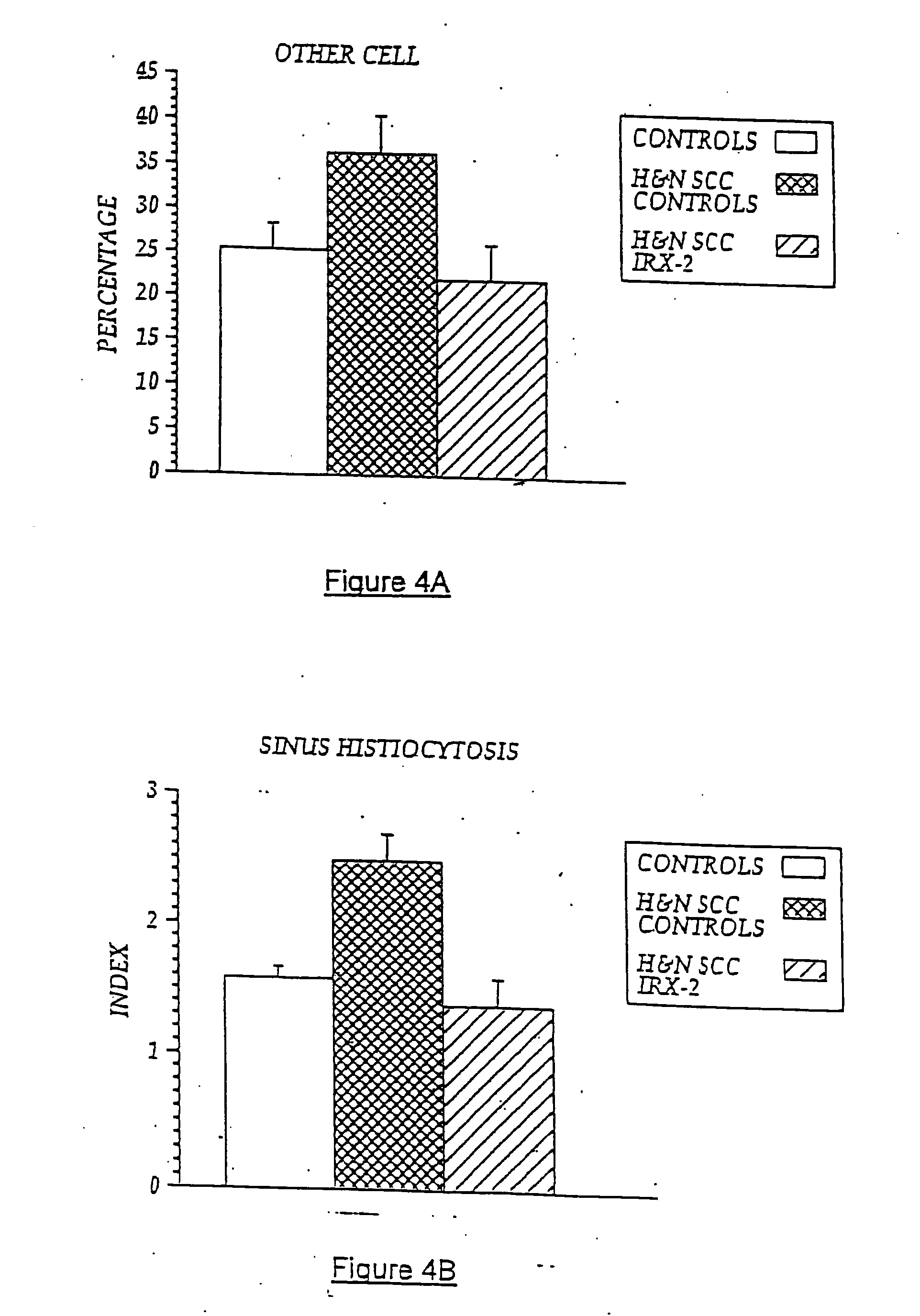
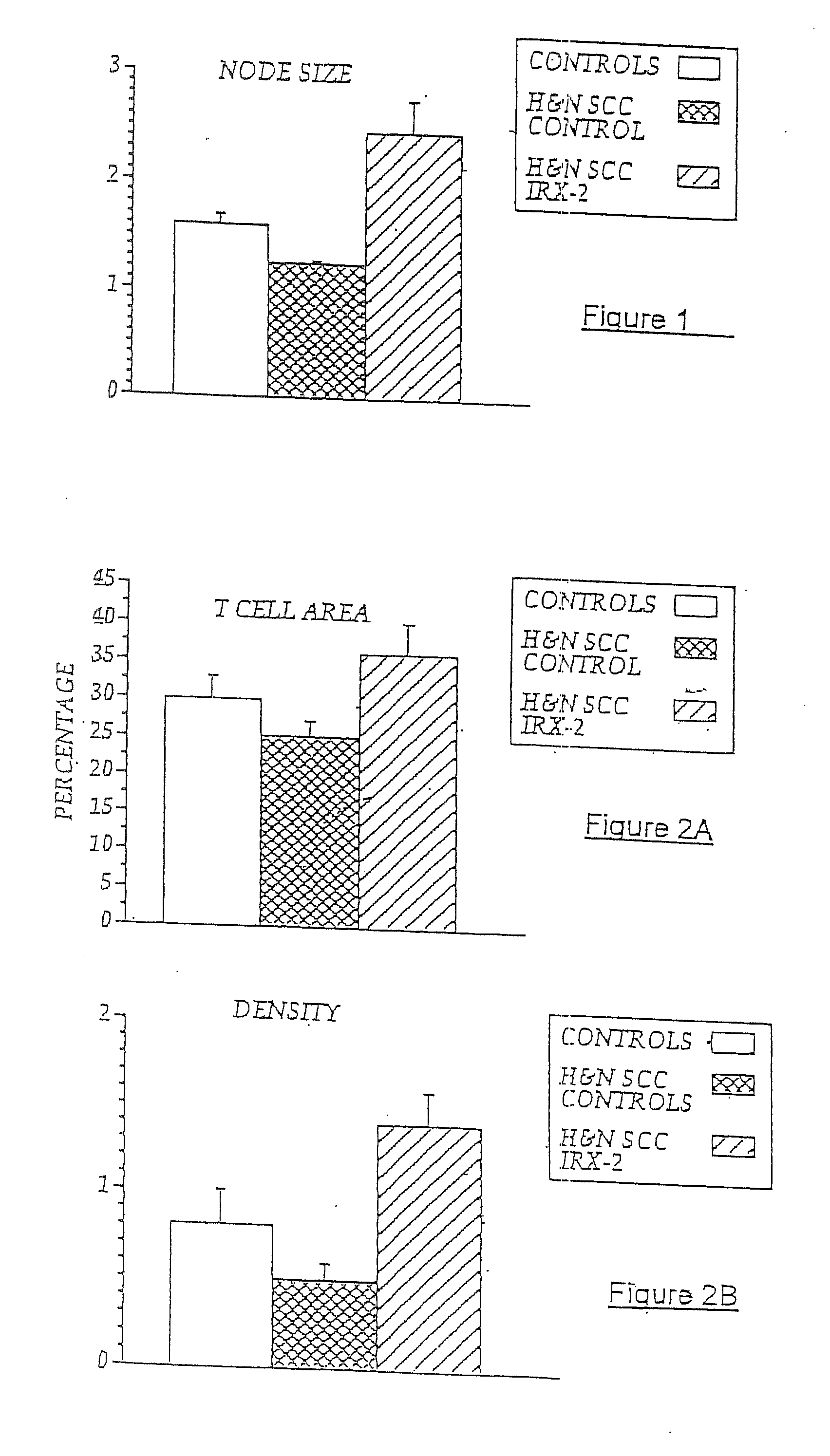
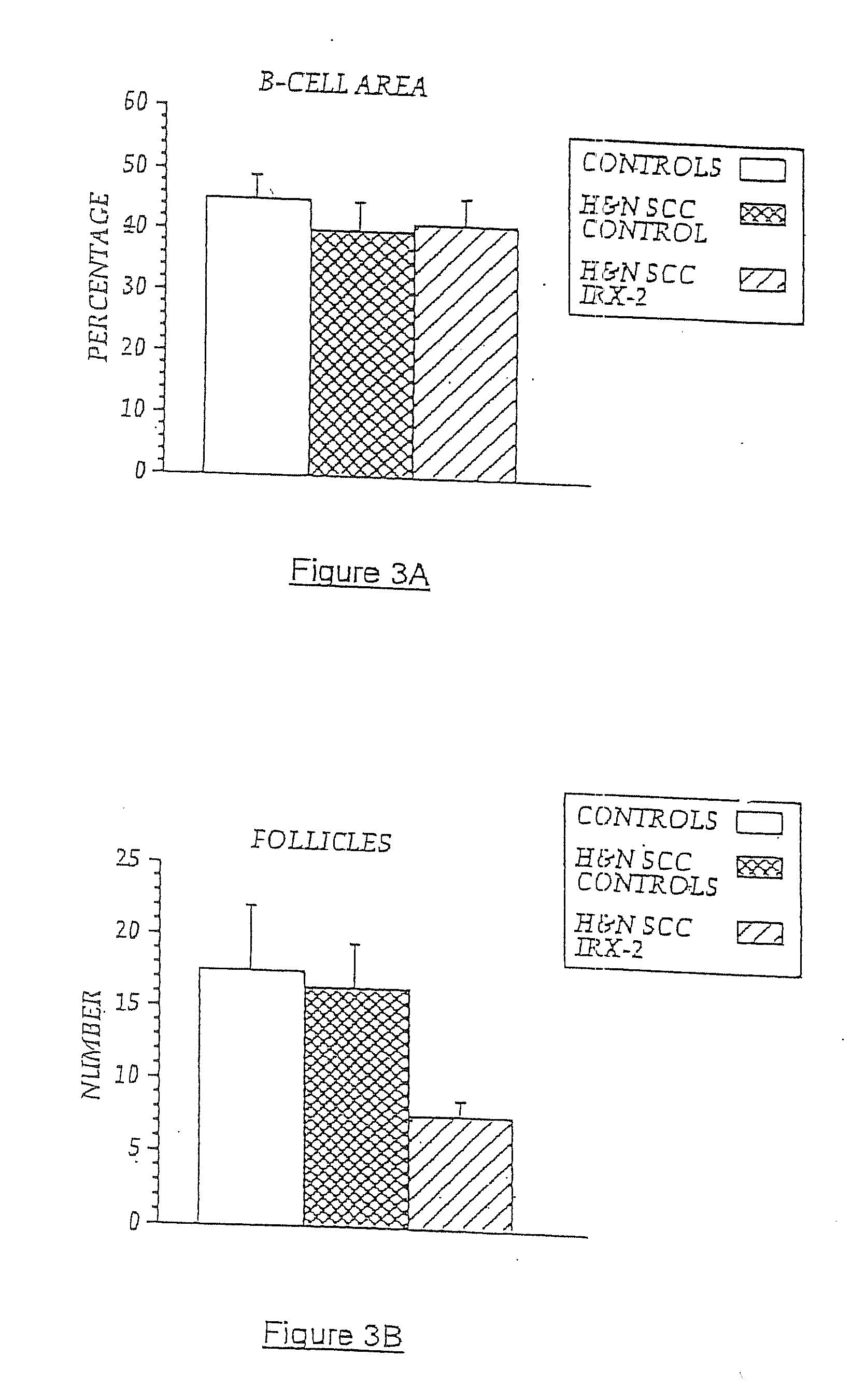
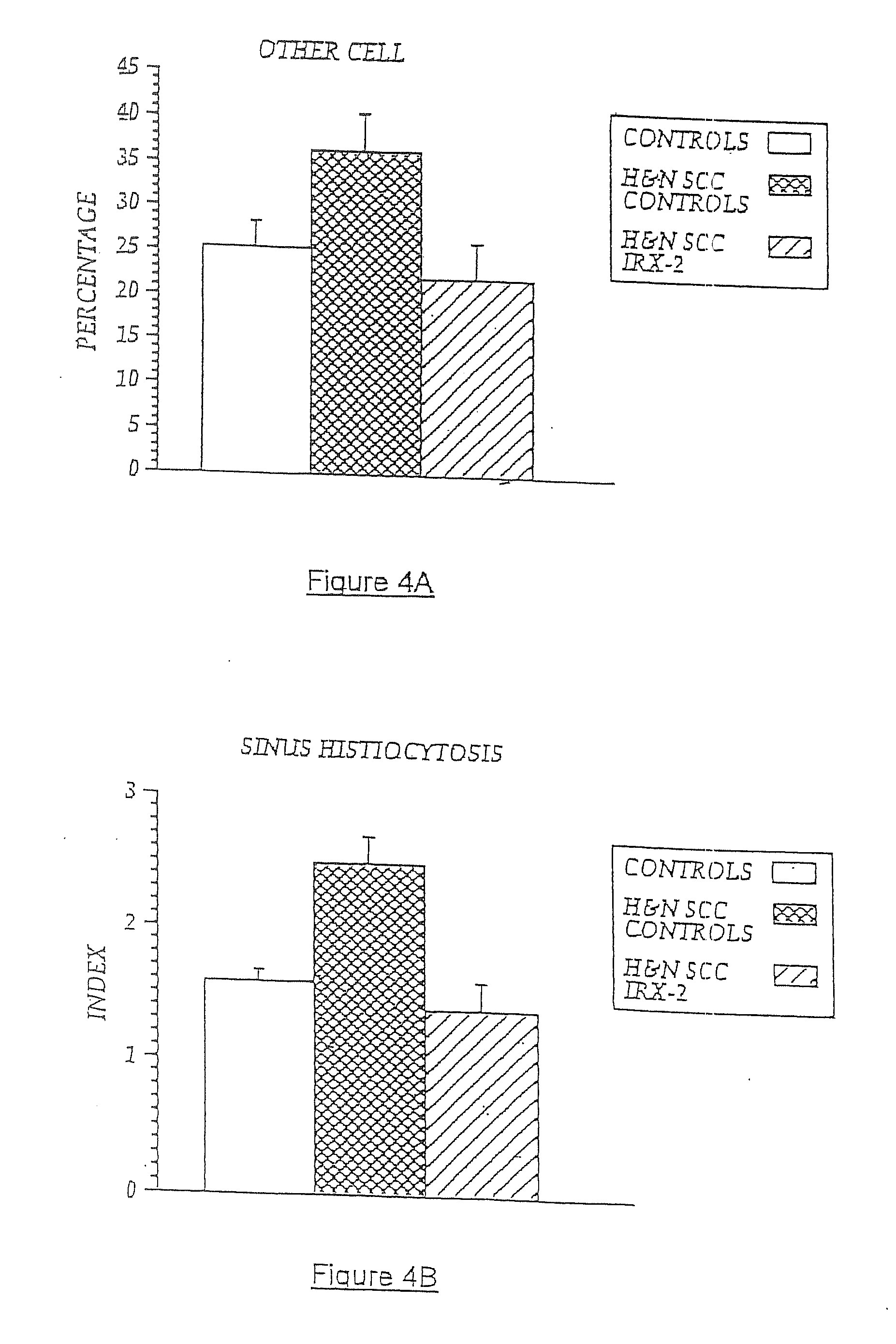
Immunotherapy for immune suppressed patients
InactiveUS20070154399A1Predictable outcomeCompounds screening/testingUltrasonic/sonic/infrasonic diagnosticsSkin allergy testEfferent
A diagnostic skin test for predicting treatment outcome, consisting essentially of an effective amount of an NCM or a T lymphocyte mitogen of muromonab-CD3. A kit for performing a skin test consisting essentially of an effective amount of an NCM or a T lymphocyte mitogen of muromonab-CD3. A method of performing a skin test on a patient, consisting essentially of the steps of administering an effective amount of an NCM or a T lymphocyte mitogen of muromonab-CD3 to skin, analyzing results of the skin test, and predicting a treatment outcome. Methods of detecting defects in monocyte or T lymphocyte function, including the steps of administering an effective amount of an NCM or T lymphocyte mitogen of muromonab-CD3 to skin, analyzing results of the skin test, and detecting at least one defect in monocyte or T lymphocyte function. A mechanism for indicating a functioning efferent or afferent limb of an immune system, including a diagnostic skin test including an effective amount of an NCM or a T lymphocyte mitogen of muromonab-CD3.
Owner:IRX THERAPEUTICS
Method for efficiently separating and expanding mesenchymal stem cells in human umbilical cord blood
ActiveCN102876630AStable growthHigh puritySkeletal/connective tissue cellsBlood/immune system cellsMedicineMesenchymal stem cell
The invention relates to an optimal method for efficiently separating and expanding mesenchymal stem cells in umbilical cord blood in cell therapy. The fact that the mesenchymal stem cells with higher differentiative capacity and smaller immunological rejection are contained in the umbilical cord blood has been proved. The establishment of an umbilical cord blood bank provides a basis for achieving transplantation of autologous cells. However, since the content of the mesenchymal stem cells in the umbilical cord blood is very low (only 0.5 to 30 mesenchymal stem cells are contained in 1*108 mononuclear cells), how to efficiently separate and expand the mesenchymal stem cells becomes a problem hindering the transformation of the mesenchymal stem cells in the umbilical cord blood to the clinical application. At present, in most situations, the mesenchymal stem cells in the umbilical cord blood are separated in virtue of adsorption capacity of the mesenchymal stem cells in a culture vessel, but the success ratio is low, and the characteristics of stem cells are difficult to maintain in an expanding process. The method comprises the steps of coating the culture vessel with protein components, adding multiple growth factors in the culture vessel in a coordination manner, and building the expansion environment of the mesenchymal stem cells in the umbilical cord blood, so as to achieve the efficient separation and expansion of the mesenchymal stem cells.
Owner:夏亮
Use of PARP-1 inhibitors for protecting tumorcidal lymphocytes from apoptosis
InactiveUS20060079510A1Strong cytotoxicityLow cytotoxicityBiocideAntipyreticMetaboliteNatural Killer Cell Inhibitory Receptors
Method and composition for protecting tumorcidal lymphocytes including cytotoxic lymphocytes and NK cells from apoptosis and down regulation are provided. The method and composition include the administration of an effective amount of a PARP-1 inhibitor to a population of cytotoxic T lymphocytes and NK cells in the presence of monocytes or macrophages. In some embodiments, the method and composition additionally include the administration of a reactive oxygen metabolite (ROM) production or release inhibitory compound. Methods of treating cancer, viral diseases, and inflammatory diseases with a PARP-1 inhibitor are likewise provided.
Owner:MAXIM PHARMA INC
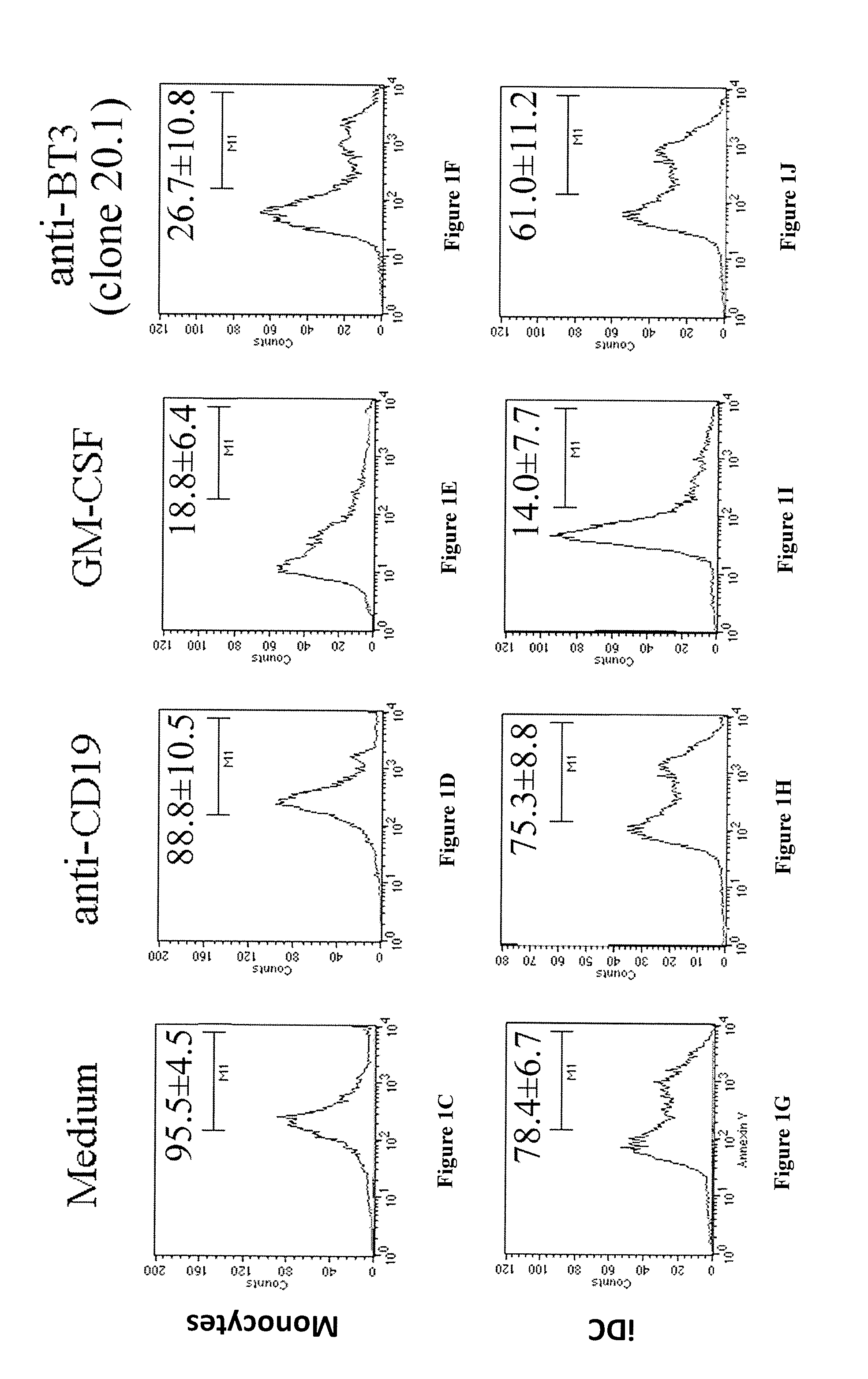
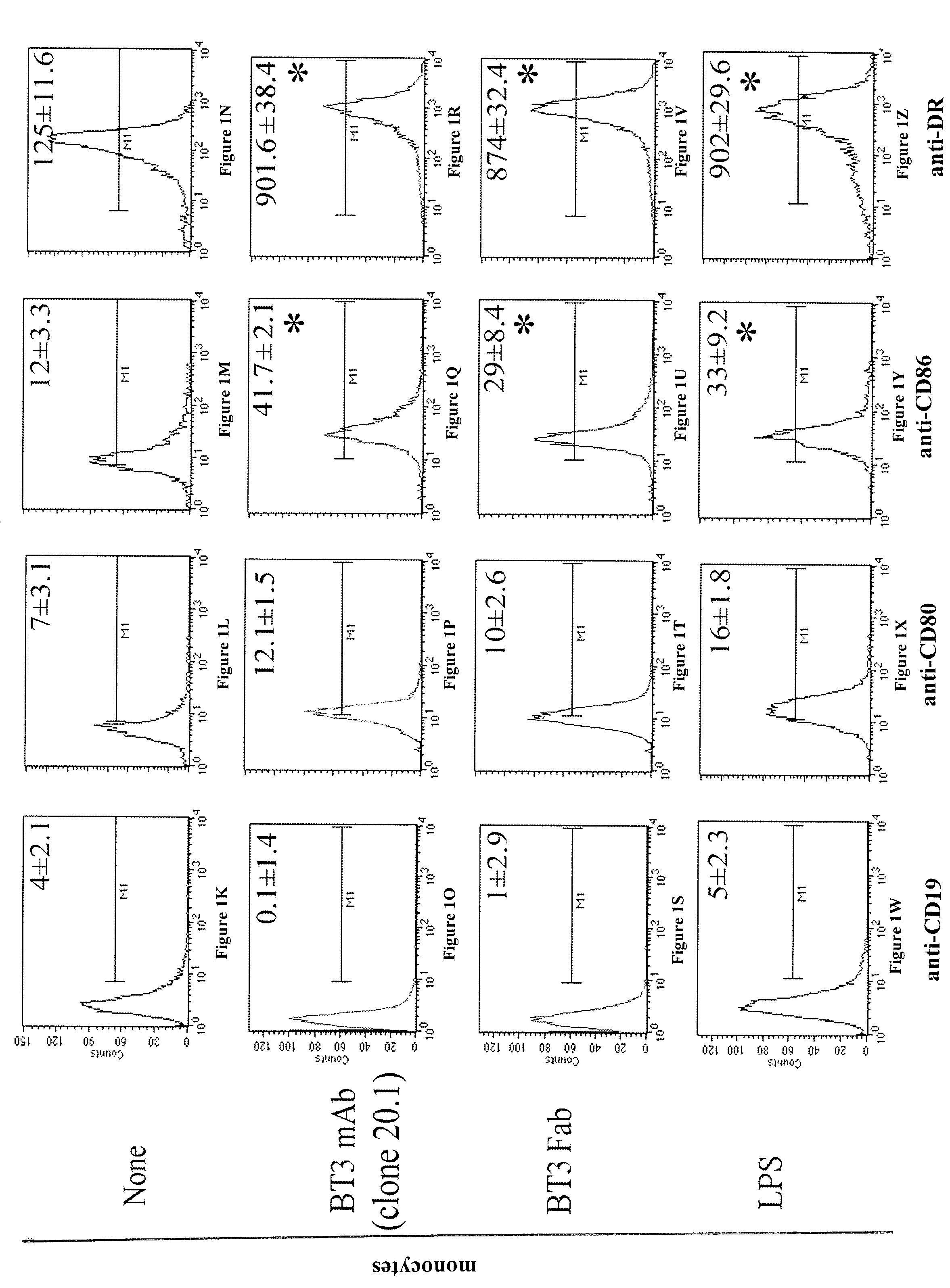
Anti-cd277 antibodies and uses thereof
InactiveUS20150353643A1High activityConducive to survivalImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDendritic cellCD28
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Apparatus and Method for Separating a Volume of Whole Blood Into At Least Three Components
InactiveUS20070203444A1Short timeOther blood circulation devicesMedical devicesInter layerWhole blood units
A method and apparatus for separating a volume of whole blood contained in a separation bag into at least three components comprising centrifuging the separation bag so as to separate therein a first inner layer comprising plasma, a second intermediate layer comprising platelets, a third intermediate layer comprising lymphocytes, monocytes and granulocytes and a fourth outer layer comprising red blood cells, transferring, into a plasma component bag connected to the separation bag, a plasma component and transferring into a mononuclear cell component bag a mononuclear cell components.
Owner:TERUMO BCT
Methods and reagents for modulating macrophage phenotype
ActiveUS20190290688A1Restore immune recognitionReduced polarization effectsOrganic active ingredientsPeptide/protein ingredientsP PHENOTYPENeonatal Fc receptor
The present invention is directed to methods of inducing a phenotypic change in a population of monocytes and / or macrophages. The method includes administering to the population of monocytes and / or macrophages, a macrophage stimulating agent coupled to a carrier molecule, wherein the carrier molecule facilitates macropinocytic uptake of the agent by monocytes and macrophages in the population and is defective in neonatal Fc receptor binding, wherein the administering induces a phenotypic change in the monocytes and macrophages in the population.
Owner:NEW YORK UNIV
Antibodies against CSF-1R
ActiveUS8263079B2Prevent dimerizationInhibit tumor growthImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDocetaxel-PNPDocetaxel
The invention provides a human antibody that binds human CSF-1R with high affinity. Antibodies of the present invention have significant advantages over the antibodies known in the art by being multifunctional: inhibiting signaling of CSF-1R, internalizing and inducing CSF-1R degradation and stimulating ADCC in cell including tumors, macrophages and monocytes. They are also shown to be effective in treating leukemia, breast, endometrial and prostate cancer alone or in combination with docetaxel, paclitaxel, Herceptin® or doxorubicin.
Owner:IMCLONE SYSTEMS
Immunotherapy for immune suppressed patients
Owner:IRX THERAPEUTICS
Injectable articular cartilage tissue repair material and its preparation method
The invention discloses an injectable articular cartilage tissue repair material and is characterized in that starting materials of the composite material contain the following components of: by weight, 100 parts of I-type collagen and 10-50 parts of hyaluronic acid, wherein oxidisability of hyaluronic acid is 20-60%. A monocyte layer in bone marrow fluid in vivo is embedded in the repair material. The thickness of the composite material is 1-5mm. Hyaluronic acid is oxidized into oxidized hyaluronic acid by the use of sodium periodate. The collagen solution, 5*PBS and a buffer are mixed at the volume ratio of 7:2:1 and the pH is adjusted to neutral. After dissolving oxidized hyaluronic acid by the use of ultrapure water, the dissolved oxidized hyaluronic acid is added into the neutral collagen solution and the mixed solution is uniformly stirred and stands in a refrigerator of 4 DEG C. The separated and extracted monocyte layer in the bone marrow fluid by a density gradient centrifugation method is uniformly mixed with the above collagen-oxidized hyaluronic acid solution. The mixed liquor is putted into a constant temperature incubator of 37 DEG C for standing or injection to the organism articular cartilage defects so as to form the composite material.
Owner:成都普川生物医用材料股份有限公司
Dedifferentiated, programmable stem cells of monocytic origin, and their production and use
The invention relates to the production of adult dedifferentiated, programmable stem cells from human monocytes by cultivation of monocytes in a culture medium which contains M-CSF and IL-3. The invention further relates to pharmaceutical preparations, which contain the dedifferentiated, programmable stem cells and the use of these stem cells for the production of target cells and target tissue.
Owner:BLASTICON BIOTECHNOLOGISCHE FORSCHUNG
Compositions and Methods of Use for Mgd-Csf in Disease Treatment
InactiveUS20070264277A1Promotes differentiationPromotes proliferationAntibacterial agentsAntimycoticsDendritic cellPregnancy
Disclosed is a newly identified secreted molecule, identified herein as “monocyte, granulocyte, and dendritic cell colony stimulating factor” (MGD-CSF), the polypeptide sequence, and polynucleotides encoding the polypeptide sequence. Also provided is a procedure for producing the polypeptide by recombinant techniques employing, for example, vectors and host cells. Additionally, procedures are described to modify the disclosed novel molecules of the invention to prepare fusion molecules. Also disclosed are methods for using the polypeptides and active fragments thereof for treatment of a variety of diseases, including, for example, cancer, autoimmune and inflammatory diseases, infectious diseases, and recurrent pregnancy loss.
Owner:FIVE PRIME THERAPEUTICS
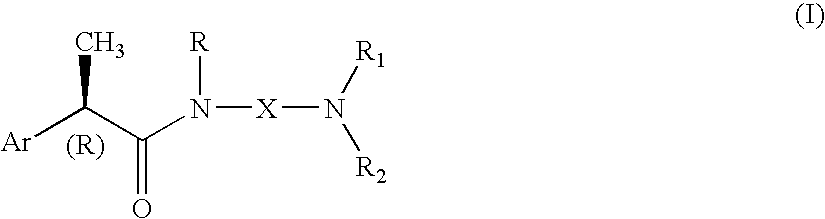
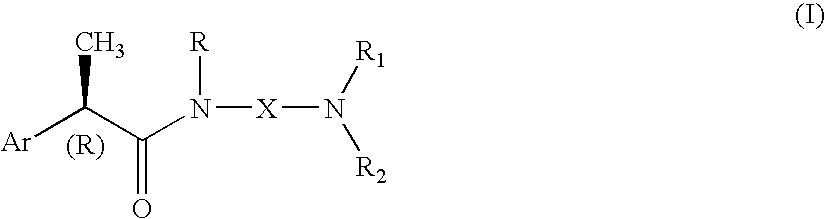
Omega aminoalkylamides of R-2 aryl propionic acids as inhibitors of the chemotaxis of polymorphonucleate and mononucleate cells
InactiveUS20050080067A1Suppression problemBiocidePeptide/protein ingredientsPropanoic acidEnantiomer
(R)-2-Arylpropionamide compounds of formula (I) are described. The process for their preparation and pharmaceutical preparations thereof are also described. The 2-Arylpropionamides of the invention are useful in the prevention and treatment of tissue damage due to the exacerbate recruitment of polymorphonuclear leukocytes (leukocytes PMN) and of monocytes at the inflammatory sites. In particular, the invention relates to the R enantiomers of omega-aminoalkylamides of 2-aryl propionic acids, of formula (I), for use in the inhibition of the chemotaxis of neutrophils and monocytes induced by the C5a fraction of the complement and by other chemotactic proteins whose biological activity is associated with activation of a 7-TD receptor. Selected compounds of formula (I) are dual inhibitors of both the C5a-induced chemotaxis of neutrophils and monocytes and the IL-8-induced chemotaxis of PMN leukocytes. The compounds of the invention are used in the treatment of psoriasis, ulcerative cholitis, glomerular nephritis, acute respiratory insufficiency, idiopathic fibrosis, rheumatoid arthritis and in the prevention and the treatment of injury caused by ischemia and reperfusion.
Owner:DOMPE FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com