Anti-cd277 antibodies and uses thereof
a technology of anti-cd277 and antibodies, which is applied in the field of anti-cd277 antibodies, can solve the problems of not being able to overcome the immunosuppressive mechanisms observed in cancer patients, and no satisfactory approach has been proven to induce potent immune responses against vaccines, etc., and achieves the effect of increasing activity and/or survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0220]Cell Cultures
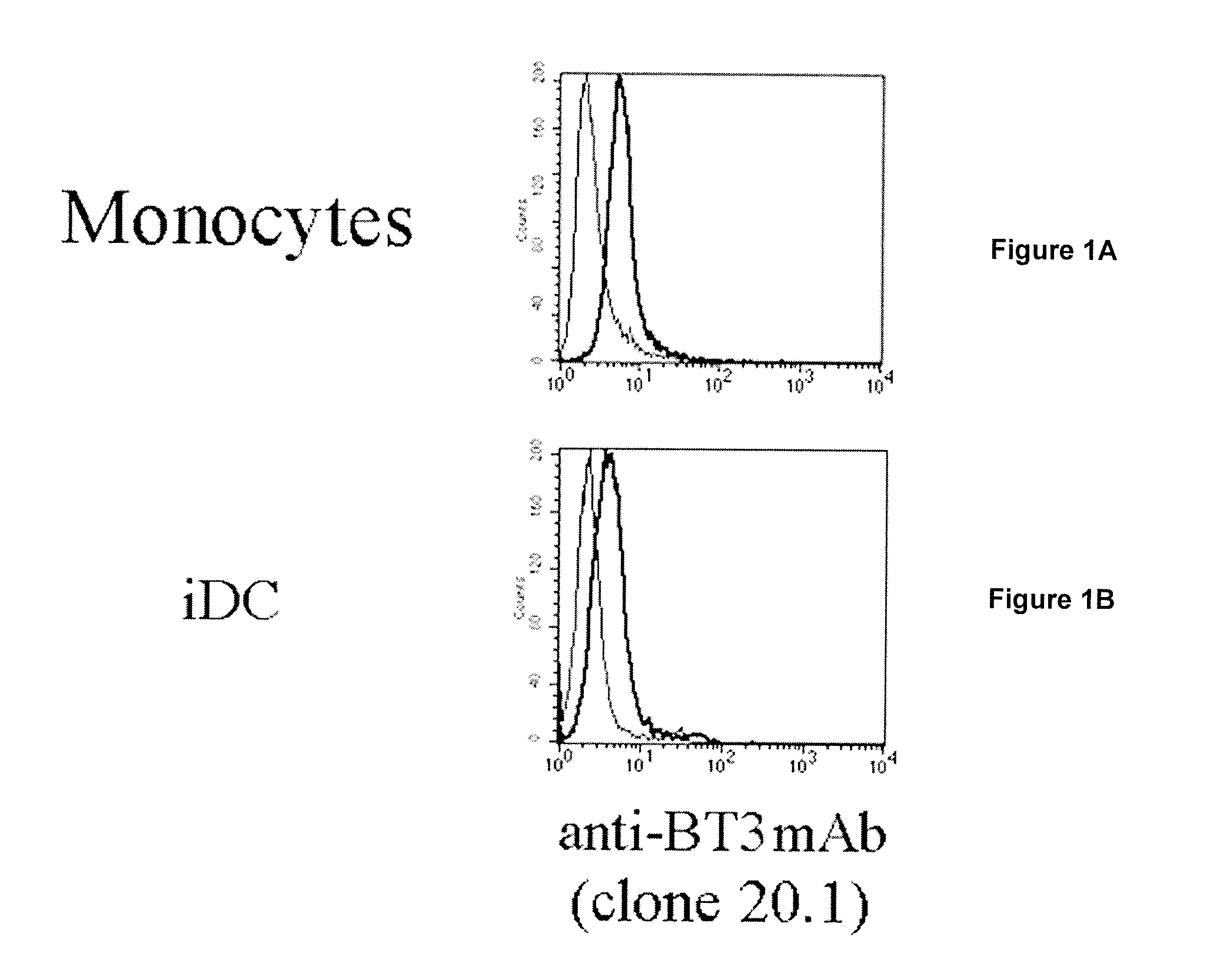
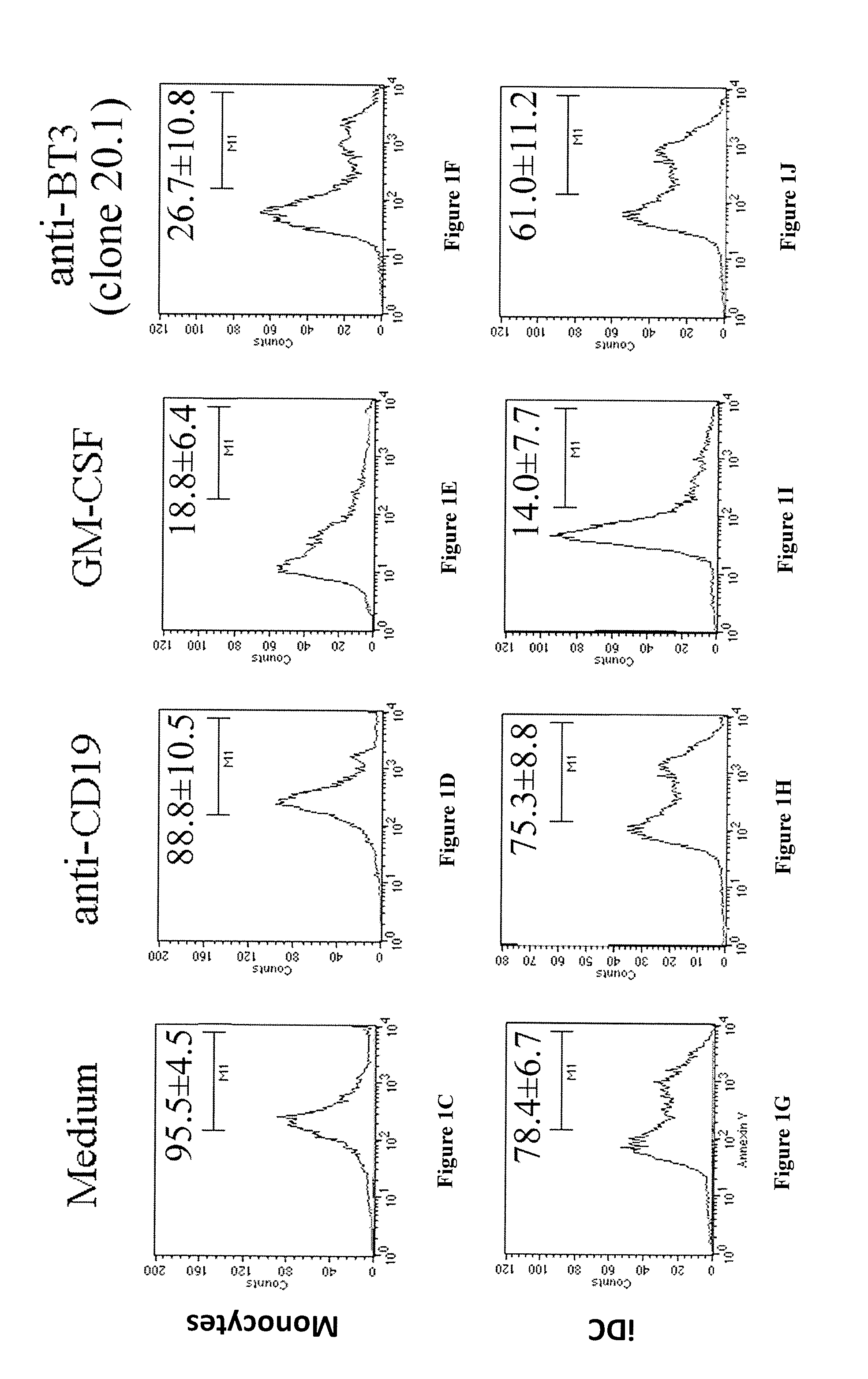
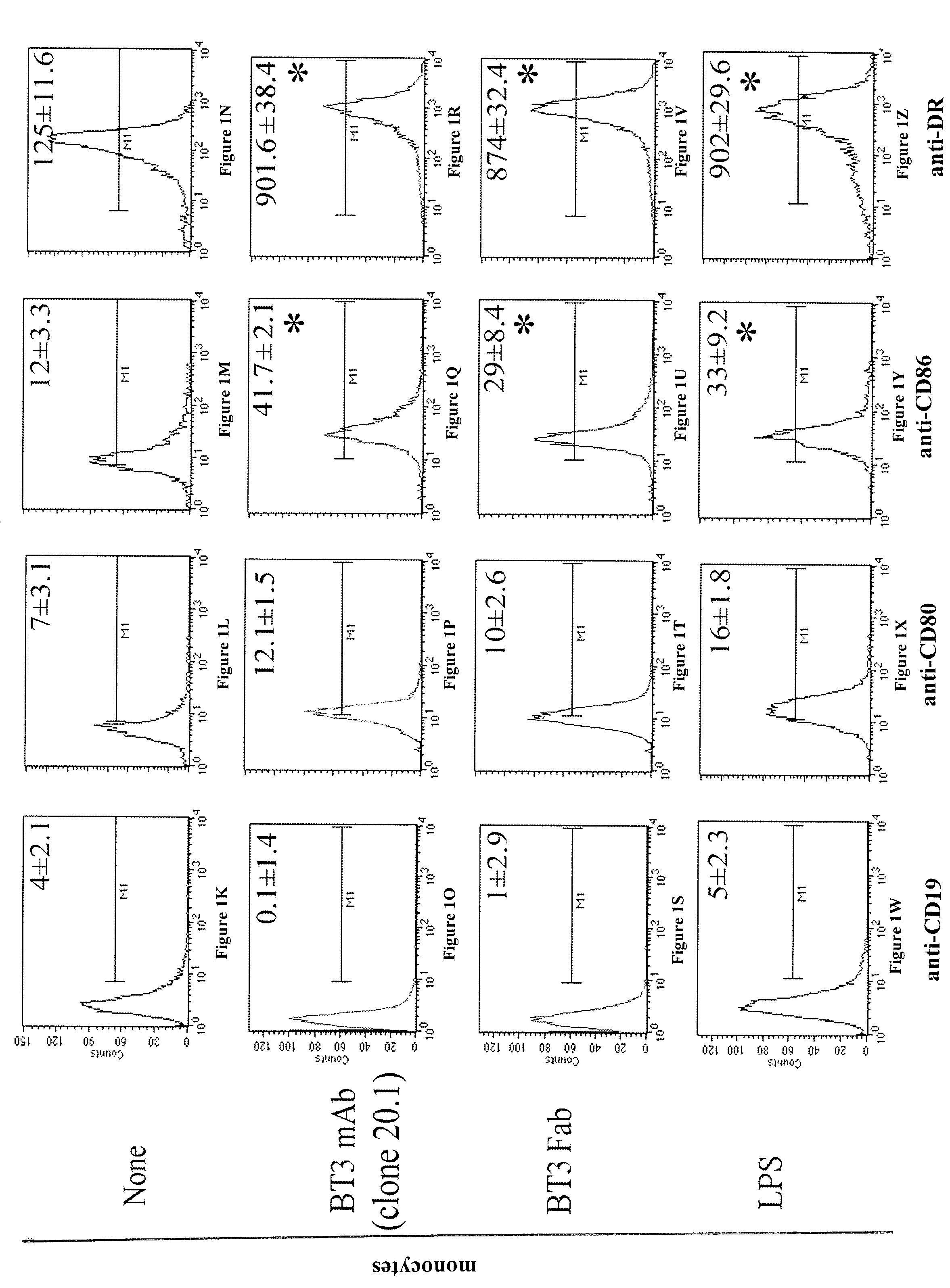
[0221]Blood samples were obtained from 5 healthy volunteers enrolled among the staff members, after informed consent. Monocytes were separated from peripheral blood mononuclear cells, and isolated using the MACS CD14 isolation kit (Miltenyi Biotech, Auburn, Calif. USA). Monocytes were cultured for 5 days with 200 ng / ml of recombinant human GM-CSF and 10 ng / ml recombinant human IL-4 (Schering-Plough Research Institute). These cells were termed immature dendritic cells (iDC). To achieve the stimulation, monocytes and iDC were cultured with 10 ng / ml of LPS, ligand for TLR4, 30 μg / ml of R848 (Sigma-Aldrich, Milano, Italy), ligand for TLR7 / 8 and with 2.5 mg / ml of poly (I:C) (Sigma-Aldrich), ligand for TLR3, and were harvested after 48 h. The cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 5 mM L-glutamine and 50 IU / ml penicillin-streptomycin (from here on referred to as complete medium).
[0222]Flow Cytometry and Antibodi...
example 2
Materials and Methods
Cell Preparation
[0240]Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteer donors provided by the “Etablissement Français du Sang” (EFS-Marseille-France) and isolated by fractionation over a density gradient of Lymphoprep© (Abcys). Human CD4+ T cells were negatively selected from isolated PBMCs by depletion of non-CD4+ T cells with magnetic beads using the T cell isolation kit II from Miltenyi Biotec®. Isolated CD4 cells were used for further experiments when purity was superior to 90%.
Generation of Monoclonal Antibodies (mAbs)
[0241]The mouse anti-human programmed death-1 (PD-1) mAB and three different clones of mouse anti-human CD277 were purified from ascites in our laboratory: Anti-PD-1 (clone PD1 3.1 with an IgG1 isotype) (ghiotto et al. Int Immunol., 2010), Anti-CD277 (clone 103.2 with an IgG2a isotype, clones 20.1 and 7.2 both with an IgG1 isotype) (compte et al.). The anti-CD277 (clone 20.1) mAb was labelled with Alexa Fluor 64...
example 3
Materials and Methods
Antibodies and Fab Fragmentation
[0279]Anti-CD277: anti-BT3-20.1 and 103.2 mAb were generated and validated as previously described [10]. Fab fragments of anti-BT3-20.1 were generated and purified with the Immunopure Fab Preparation Kit following the manufacturer's recommendation (Pierce). Protein purity was assessed by nonreducing SDS-PAGE.
Construction of Phylogenetic Trees
[0280]Phylogenetic analyses were performed using the automated genomic annotation platform FIGENIX (FIGENIX Annotation Platform: [http: / / figenix2.up.univmrs.fr / Figenix / index.jsp]) to retrieve sequences and alignments and perform phylogenetic reconstruction. The pipeline used applied three different methods of phylogenetic tree reconstruction, i.e. Maximum Parsimony [38], Maximum likelihood [39] and Neighbour Joining [40], and a midpoint rooted consensus tree was built. Bootstrapping was carried out with 1000 replications. Bootstrap values are reported for each method (for a detailed descriptio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com