System for detecting bacteria in blood, blood products, and fluids of tissues
a tissue and blood technology, applied in the field of tissue and blood products, can solve the problems of inability to use donor blood and blood products or donor organs, and inability to quickly and efficiently detect the presence of contaminating bacteria in blood or blood products, etc., to achieve rapid propagation rate, short shelf life, and increase the amount of contaminating bacteria presen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
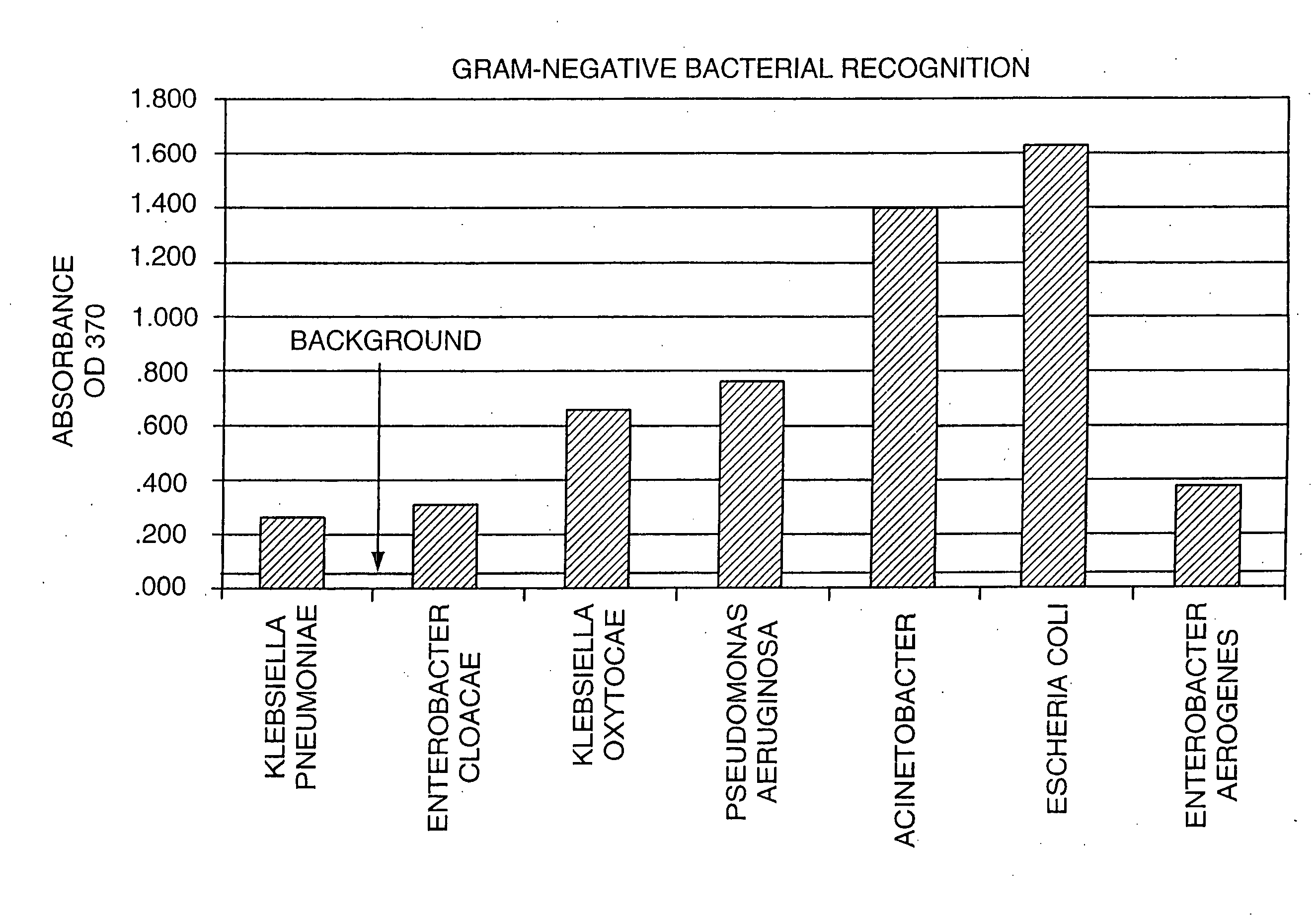
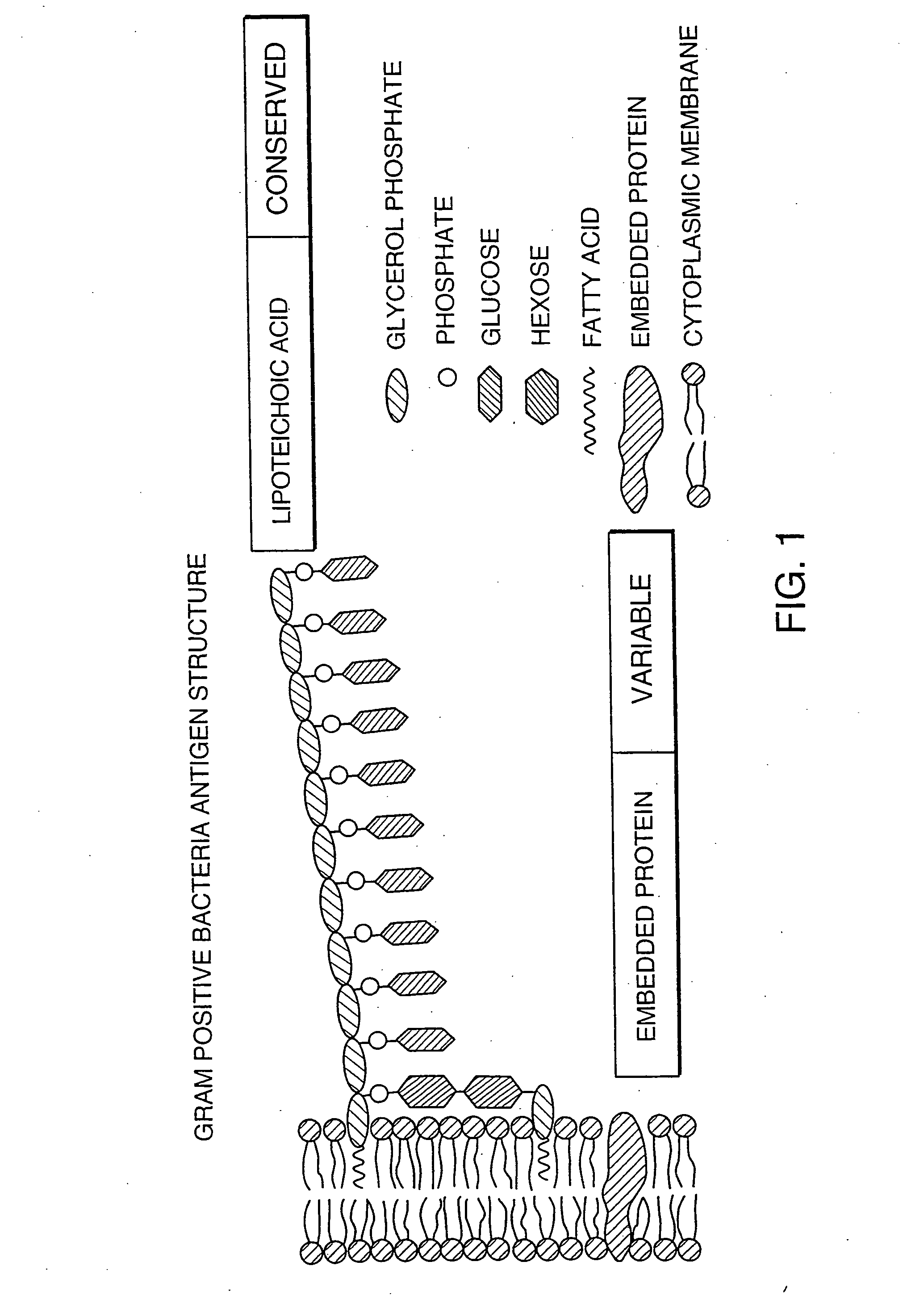
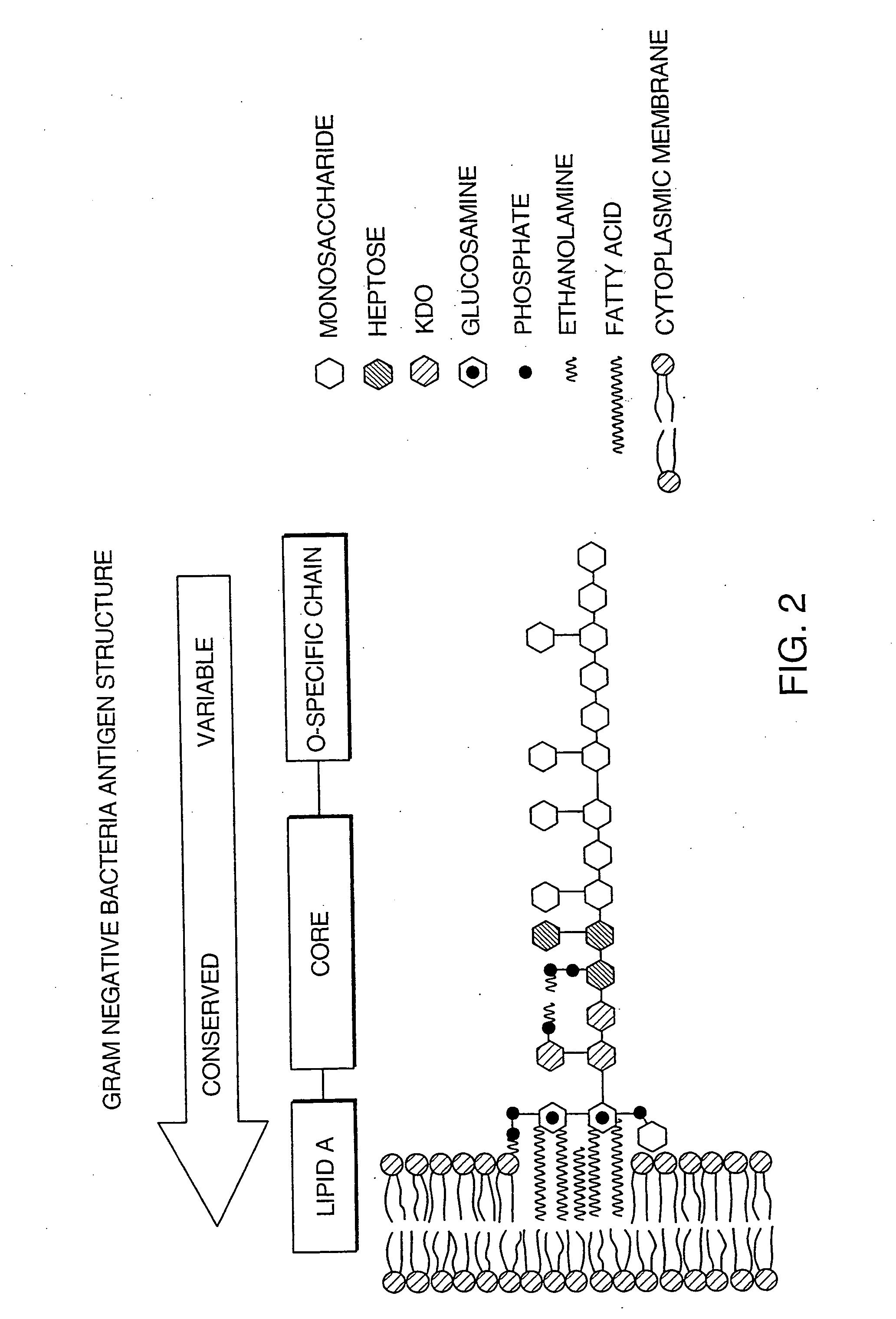
[0142]A bacterial detection method of the invention can follow a conventional particle-based immunoassay format. In this approach, binding agents that specifically bind to an antigenic region on the bacterial cell wall can be utilized as immunological targets and can form the basis for detecting the bacteria in the blood products. A schematic of the device used in this example is shown on FIGS. 3A-3D. In one embodiment, the binding agents that specifically bind to Gram-positive bacteria, Gram-negative bacteria, or both, are pooled together, and then roughly divided, such that the two populations of binding agents each comprises a mixture of binding agents. One population is bound to the membrane at the base of the device shown in FIGS. 3A-3D. The second population is used to coat brightly dyed latex particles (i.e., the latex particles coated with the binding agent have been impregnated with a dye so that they are intensely colored).
[0143]In another embodiment, the same binding agen...
example ii
[0149]In another embodiment of the method described in Example I, the latex-bound binding agents are binding agents that specifically recognize either Gram-negative bacteria or Gram-positive bacteria, or both. However, each of these anti-bacteria binding agents has an epitope that is common to all binding agents. In this example, all of the anti-bacteria binding agents are murine antibodies. The membrane bound binding agents do not specifically bind bacteria; rather, they specifically bind the murine determinants on the constant region of the murine anti-bacteria antibodies. These anti-mouse binding agents are called secondary binding agents, because they do not specifically bind the bacteria, but rather, specifically bind the binding agent that is specifically bound to the bacteria.
example iii
[0150]An agglutination assay is yet another method to detect clinically relevant amounts of bacteria in blood, a blood product, or fluid from a donor tissue. In this example of an agglutination assay, latex particles coated with binding agents that specifically bind to bacterial species are employed. These latex particles agglutinate in the presence of clinically relevant amounts of bacterial species. A positive reaction is indicated by the development of an agglutinated pattern. In a negative reaction the latex does not agglutinate and the milky appearance remains substantially unchanged.
[0151]In this method, a test device such as that shown on FIGS. 4A-4D is employed. A sample extracted under sterile conditions from a donor blood or blood product is applied to the test device. Alternatively, a sample extracted from a fluid in which a donor organ or tissue is stored may be applied to the test device. The sample may be tested straight (i.e., undiluted or untreated), diluted with a b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com