Ectodysplasin (Eda)-containing medicine for treating ocular surface diseases and corneal diseases
A technology for exogenous proteins and corneal diseases, which is applied in the field of drugs for the treatment of ocular surface and corneal diseases, can solve problems that have not been reported, and achieve the effect of promoting repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
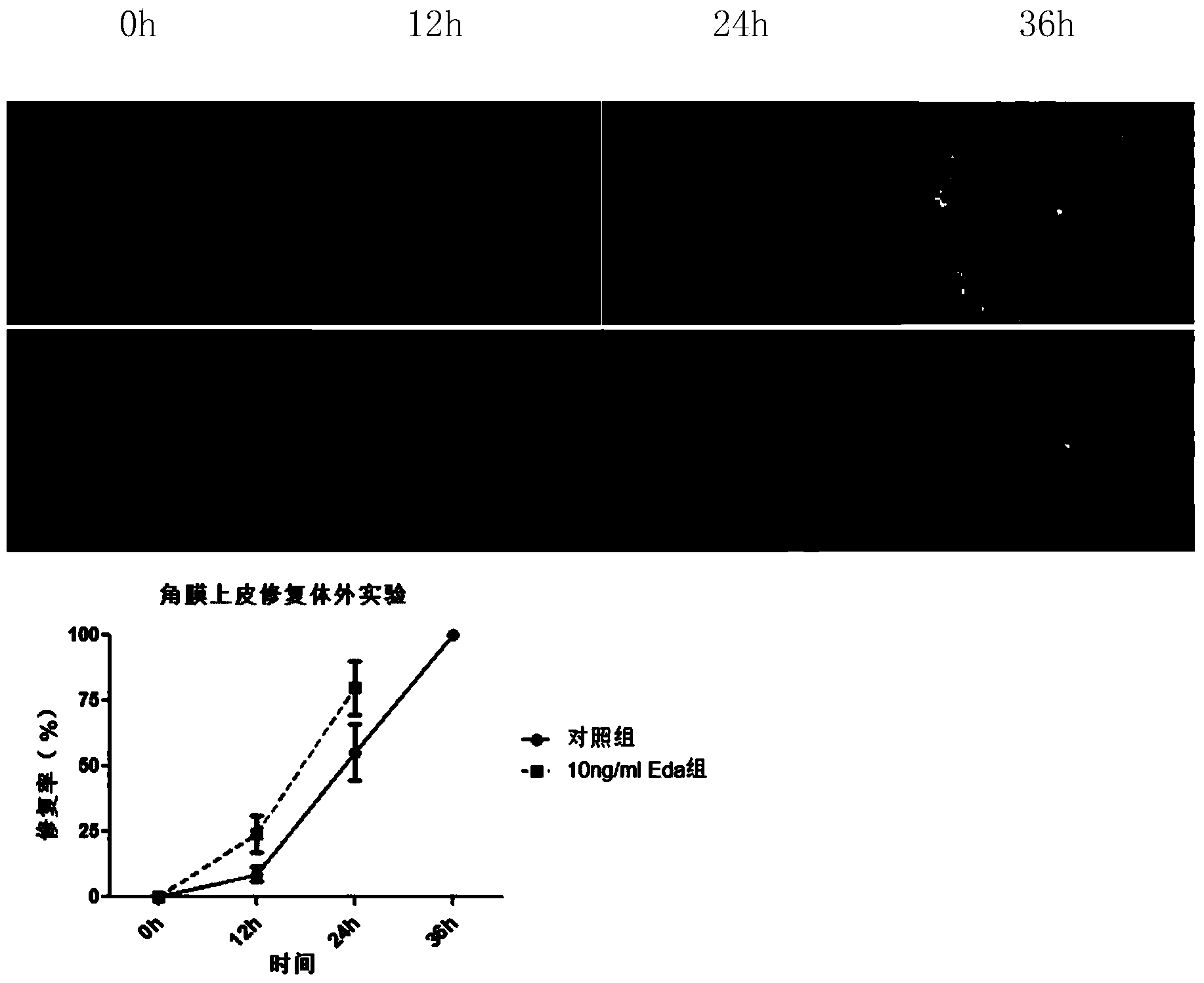
[0020] Take 4 C57 mice of the same age, remove the central corneal epithelium, remove the eyeballs, put the eyeballs into 1% FBS for culture, and randomly divide them into Eda administration group and control group, add 10ng The Eda protein of / ml is administered once in 12h, and is administered continuously for 36h, and at 0h, 12h, 24h, and 36h, the cornea is stained with sodium fluorescein, measures its damage and repair area, and calculates the corneal epithelial repair rate (specifically Such as figure 1 shown). It can be concluded that Eda protein can promote the repair of corneal epithelium.
Embodiment 2
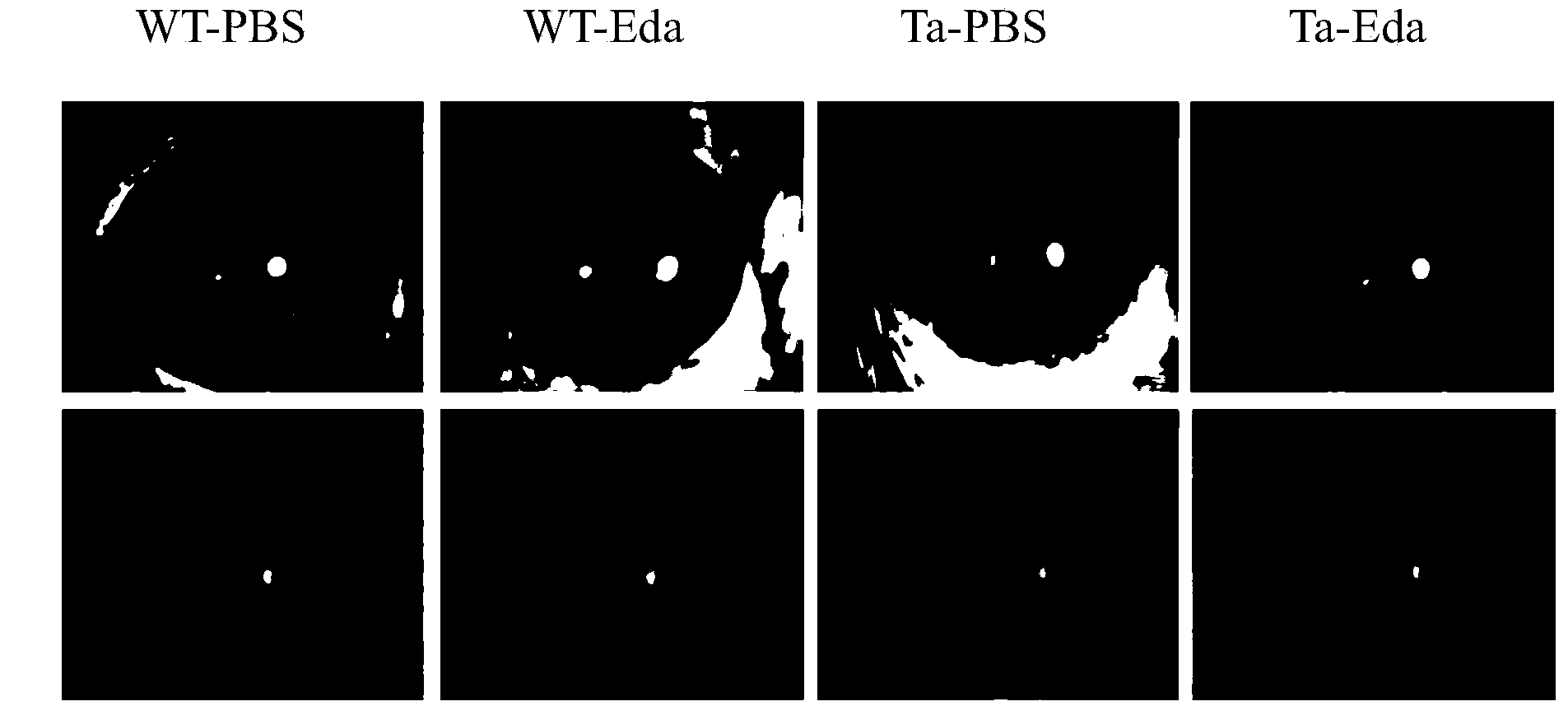
[0022] Select 6 wild-type (WT) mice and 6 Eda gene mutant (Ta) mice 5 weeks after birth, and give 7ul of Eda eye drops with a concentration of 10ng / ml to the right eye of the mice each time, once every 6 hours , administered continuously for three weeks, the same dose of Eda eye drops solvent PBS was administered to its left eye. three weeks later, if figure 2 As shown, compared with the Ta-PBS group, the fluorescein sodium staining of the cornea in the Ta-Eda group was significantly reduced, and the ocular surface lesions were improved; in both the WT-Eda group and the WT-PBS group, no corneal abnormalities were seen. It can be concluded that the Eda protein has no toxicity to the cornea, and has the effect of treating ocular surface diseases in Eda gene mutant mice.
Embodiment 3
[0024] The preparation method of eye drop, comprises the steps:
[0025] 1. Precisely weigh 0.001-1000mg of natural Eda protein or Eda recombinant protein, 0.1-50g of pH regulator, 0.1-50g of isotonic agent, and 0.01-5g of stabilizer;
[0026] 2. First fully dissolve the natural Eda protein or Eda recombinant protein with 1-50 times the amount of dissolving agent, stir and mix, adjust the pH range: 7.0-8.0, and dilute to 1000ml with water for injection to obtain the medicinal solution;
[0027] 3. In a class 100 environment, the liquid medicine obtained in step 1 can be filtered through a microporous membrane for 1 to 5 times;
[0028] 4. In a class 100 environment, fill the liquid medicine into a single-dose packaging container, seal it, and obtain the finished product.
[0029] The dissolving agent is propylene glycol and / or hydroxypropyl beta cyclodextrin; the pH regulator is sodium dihydrogen phosphate, disodium hydrogen phosphate, potassium dihydrogen phosphate, sodium h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com