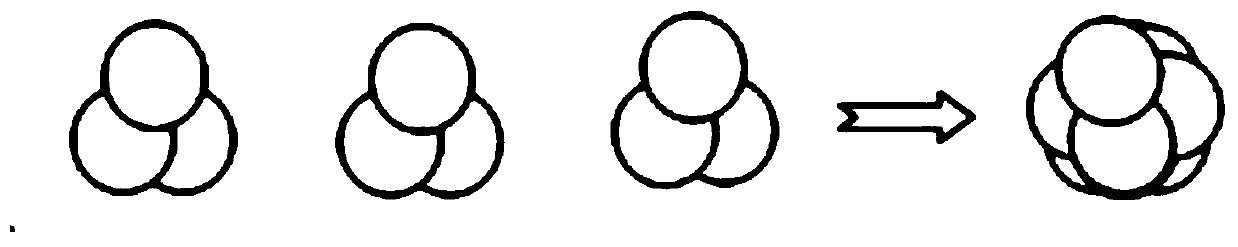Medium, corneal stromal slice prepared by medium and preparation method
A technology of corneal stroma and culture medium, applied in biochemical equipment and methods, culture process, tissue culture, etc., which can solve the problems of complex production process, difficulty in wide application, and shortage of tissue sources, and achieve good growth and good biocompatibility Effects of stability, good biocompatibility and cell affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of Decellularized Corneal Lens
[0062] (1) Disinfection: Soak the corneal stromal lens with the accurate thickness of the cells in physiological saline or phosphate buffer solution containing penicillin at a concentration of 0.01-0.1 mg / ml and streptomycin at a concentration of 0.05-0.5 mg / ml for 1 -5h, then rinse with 0.9% normal saline or phosphate buffer;
[0063] (2) Decellularization treatment: rinsing with phosphate buffer for 3 times, soaking in sodium chloride solution with a concentration of 1.5mol / L for 48 hours, changing the solution every 24 hours, and then using 0.9 Rinse with % normal saline or phosphate buffer for 48 hours, then soak in phosphate buffer for 72 hours, and change the solution every 24 hours;
[0064] (3) Sterilization: irradiate with gamma rays, and the irradiation dose is 20-30 kGy.
Embodiment 2
[0065] The preparation of embodiment 2 cornea extract
[0066] 1. Take some fresh porcine corneas and remove the epithelium and endothelium.
[0067] 2. After washing with PBS, soak in a mixture containing 50% glycerol and 50% DMEM, and store at -80°C to inactivate the corneal tissue.
[0068] 3. After thawing, wash with PBS, dry and weigh.
[0069] 4. Put some corneal stroma with known total weight into a mortar, pour liquid nitrogen into it, and grind until it becomes a fine powder.
[0070] 5. Add sterile, 4°C PBS in an amount of 5-10ml / g, and homogenate.
[0071] 6. Shake the suspension at 300rpm at 4°C for 48h, then centrifuge at 20000-25000g for 20-30min to remove undissolved debris.
[0072] 7. Aspirate the supernatant, filter it with a 0.22 μm filter head, measure the concentration of the extract by the BCA method, and store it at -80°C after aliquoting.
Embodiment 3
[0073] The preparation of embodiment 3 corneal stroma sheet
[0074] The corneal lens used in this example is prepared from Example 1.
[0075] Take a piece of corneal lens, and coat the surface with a mixed solution of 2 thrombin with a concentration of 50U / ml and calcium chloride with a concentration of 40mmol / L, and then coat with 2ul of fibrinogen with a concentration of 20mg / ml. Then quickly put another lens on top of the first lens, repeat the above steps, according to figure 1 3 corneal lenses are laminated to form the corneal lens layer of the present invention. The prepared corneal lens layer was prepared with DMEM / F12 medium containing 7.5 μg / ml corneal extract, 15 μmol / L Y-27632, 15ng / ml ITS, 20ng / ml FGF, 2mmol / L ascorbic acid, and 0.5vol% fetal calf serum Cultivate for 24h. The appearance of the prepared corneal stroma sheet sample is shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com