Formula of medicament for treating non-infectious ocular inflammations, and inhibiting corneal neovascularization and anti-rejection reaction generated after corneal grafting
A corneal neovascularization, non-infectious technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of easy inactivation, reduced dosage, and high external environment requirements. To achieve the effect of easy preservation, improved activity and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
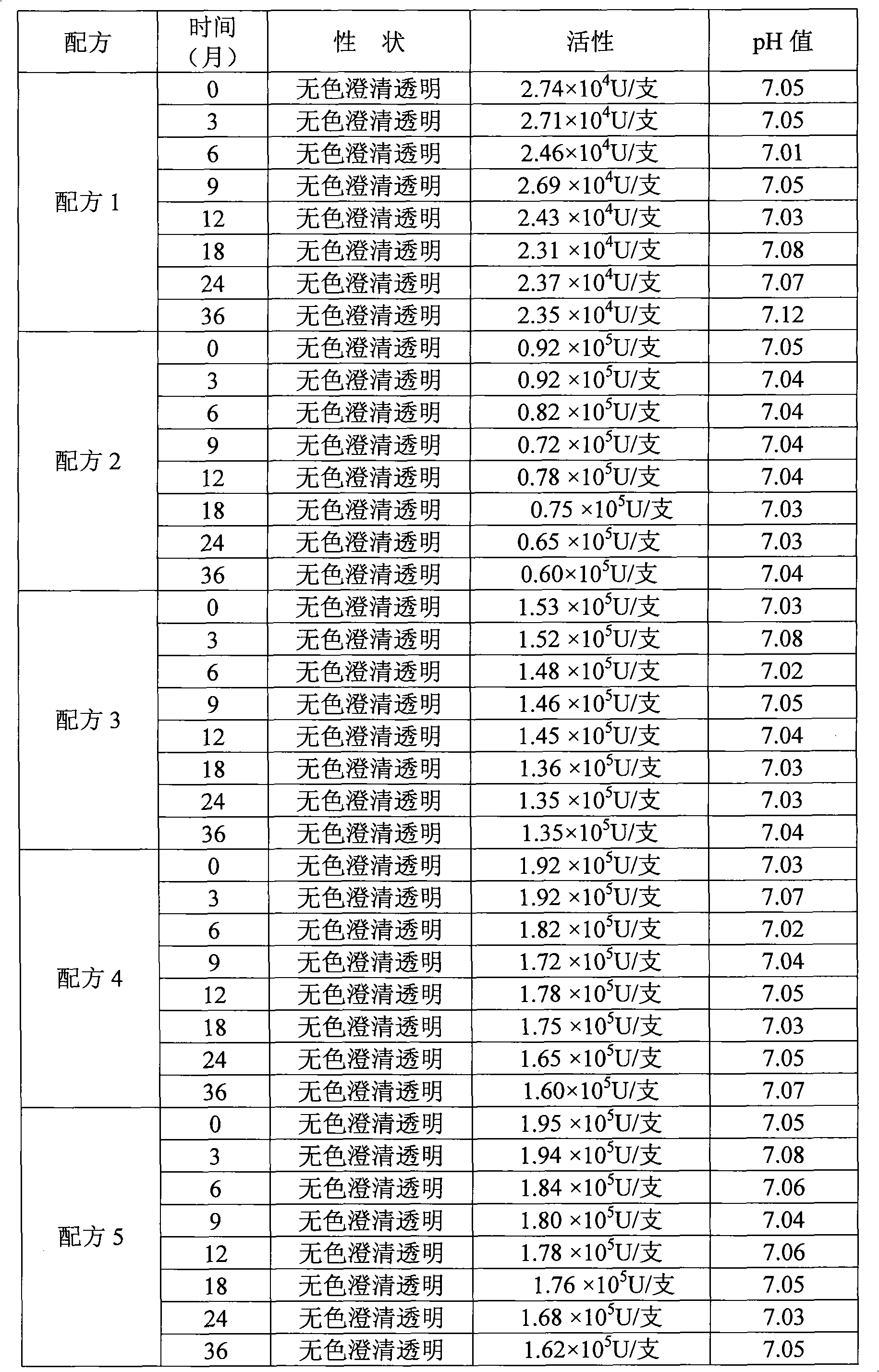
[0030] Embodiment 1 chooses that isotonic agent is glycerin and isotonic agent is the test result comparison of mannitol
[0031] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0032] Formula 2 Glycerin replaces mannitol, and the weight of glycerin is 10g.
[0033] Formula 3 Glycerin replaces mannitol, and the weight of glycerin is 20g.
[0034] Formula 4 Glycerin replaces mannitol, and the weight of glycerin is 30g.
[0035] Formula 5 glycerin replaces mannitol, and the weight of glycerin is 40g.
[0036] Choose every kind of formula solution to pack into 50 bottles, each bottle is 10 milliliters, and the test results are averaged.
[0037]
[0038] As can be seen from the results in Table 1,...
Embodiment 2
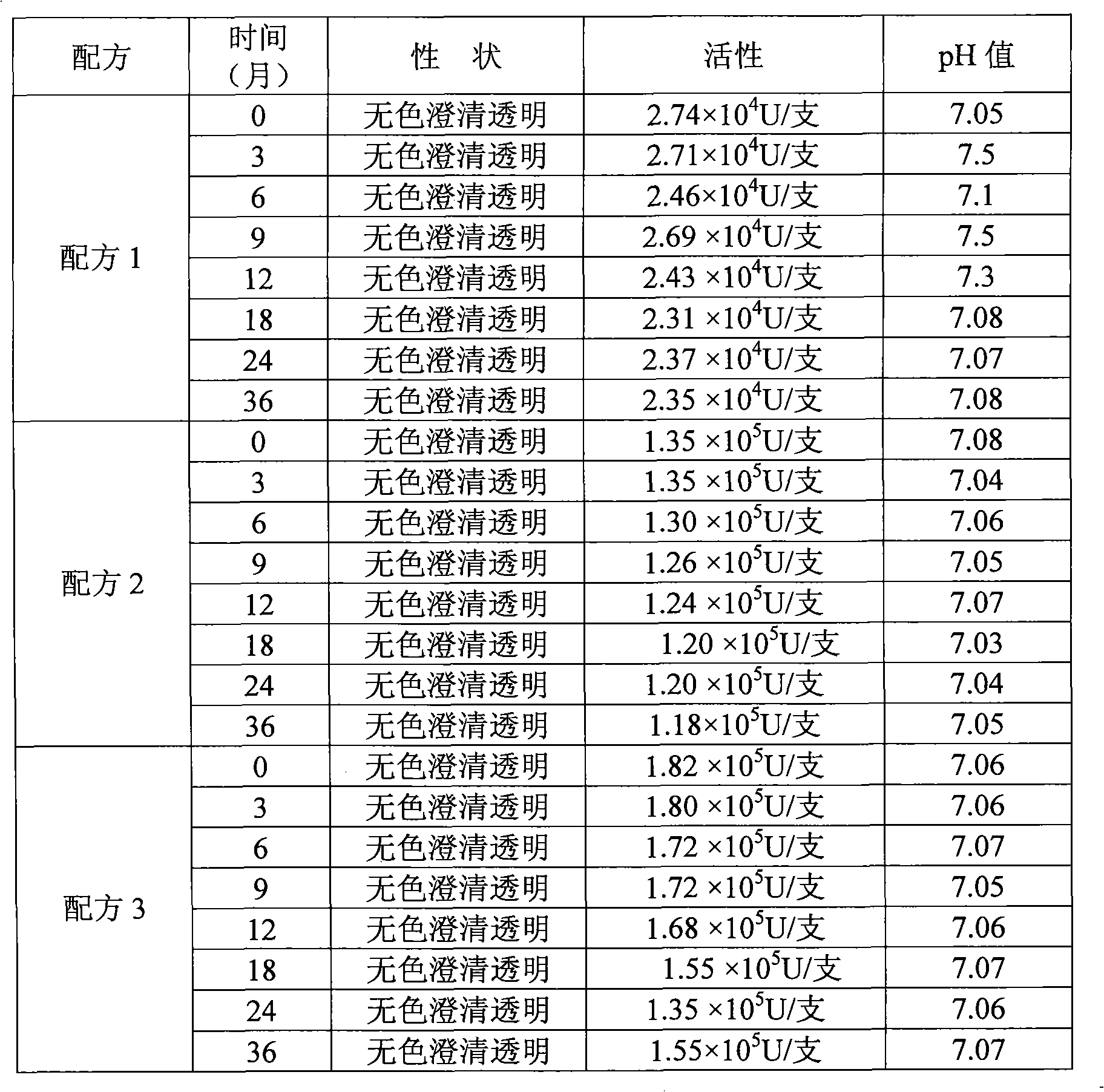
[0039] Embodiment 2 adopts the test result of sodium citrate and citric acid combination and disodium hydrogen phosphate and potassium dihydrogen phosphate contrast
[0040] Table 2 Comparison of different test results of buffer
[0041]
[0042] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0043] Formula 2 uses a combination of sodium citrate and citric acid as a buffer to replace the combination of disodium hydrogen phosphate and potassium dihydrogen phosphate, and the sodium citrate and citric acid are 40g and 20g respectively.
[0044] Formula 3 uses a combination of sodium citrate and citric acid as a buffer to replace the combination of disodium hydrogen phosphate and potassium dihydrogen...
Embodiment 3
[0047] Embodiment 3 adds preservative comparative test
[0048] Table 3 Adding preservatives and comparing the experimental results without adding
[0049]
[0050] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0051] Formula 2 adds 0.2 g of ethyl p-hydroxybenzoate on the basis of formula 1.
[0052] Choose every kind of formula, and solution is packed into 50 bottles, and every bottle is 50 milliliters, and test result is averaged.
[0053] From the test results in Table 3, it can be known that adding a preservative can better keep the recombinant human interleukin-1 receptor antagonist in a sterile and qualified state, and keep its activity stable for a longer period of time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com