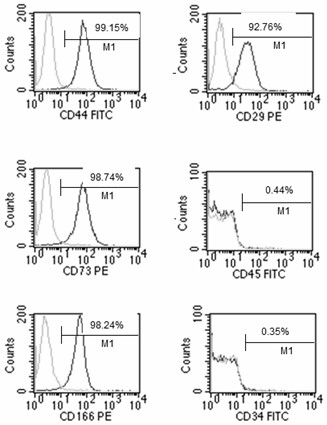
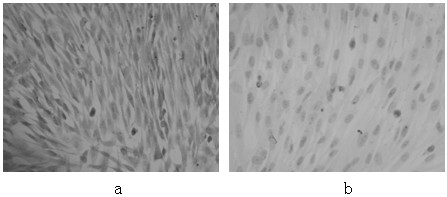
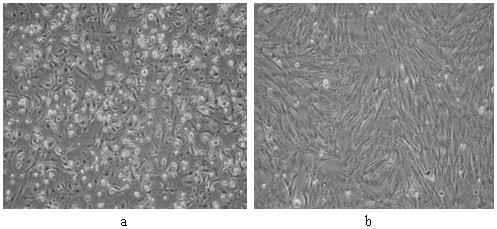
Method for sequential culture of human umbilical cord blood mesenchymal stem cells by using two culture media
A technology of mesenchymal stem cells and human umbilical cord blood, applied in the field of cell engineering and biomedicine, can solve the problems of high economic cost, reduced success rate of hUCB-MSCs culture, differential adherence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 of the present invention: a method for sequentially culturing human umbilical cord blood mesenchymal stem cells in two culture media, comprising the following two steps:
[0051] (1) Isolation of mononuclear cells: the aseptically collected heparin anticoagulated umbilical cord blood was mixed with D-PBS buffer (take KCl 0.20g, KH 2 PO 4 0.20g, NaCl 8.00g, NaCl 2 HPO 4 ·7H 2 (O2.16g, add ultrapure water to 1000ml, adjust pH to 7.2-7.4, autoclave, cool and store in 4°C refrigerator) After dilution and mixing, add diluted blood to lymph On the cell separation medium (density 1.077g / ml) (purchased from Pharmacia, USA), after centrifugation, absorb the middle buffy coat, wash with D-PBS buffer containing 2mmol / L EDTA, and add 0.83% ammonium chloride to lyse For red blood cells, wash the cells with PBS buffer containing 2% FBS, centrifuge and suspend the pellet in DMEM / F12 medium, stain with 0.4% trypan blue, and observe the cell viability.
[0052] (2) Cul...
Embodiment 2
[0053] Embodiment 2 of the present invention: a method for sequentially culturing human umbilical cord blood mesenchymal stem cells in two culture media, comprising the following two steps:
[0054] (1) Separation of mononuclear cells: Dilute the aseptically collected heparin-anticoagulated umbilical cord blood with D-PBS buffer, mix well, and add the diluted blood to the lymphocyte separation medium (density 1.077 g / ml), after centrifugation, absorb the middle buffy coat layer, wash with D-PBS buffer containing 2mmol / L EDTA, add 0.83% ammonium chloride to lyse the red blood cells, wash the cells with PBS buffer containing 2% FBS, After centrifugation, the precipitate was suspended in DMEM / F12 medium, stained with 0.4% trypan blue, and the cell viability was observed.
[0055] (2) Culture: when the cell viability is >95%, the cell density is 5×10 6 cells / ml, inoculate isolated mononuclear cells in T25 culture flasks, inoculate 2-3 T25 culture flasks with cells obtained from 1...
Embodiment 3
[0056] Embodiment 3: A method for sequentially culturing human umbilical cord blood mesenchymal stem cells in two media, comprising the following steps:
[0057] (1) Separation of mononuclear cells: Dilute the aseptically collected heparin-anticoagulated umbilical cord blood with D-PBS buffer, mix well, add the diluted blood to the lymphocyte separation medium at a volume ratio of 1:1, and centrifuge Aspirate the middle buffy coat, wash with D-PBS buffer containing 2mmol / L EDTA, then add 0.83% ammonium chloride to lyse the red blood cells, wash the cells with PBS buffer containing 2% FBS, centrifuge, and culture the pellet with DMEM / F12 base suspension, stained with 0.4% trypan blue, and observed cell viability.
[0058] (2) Culture: when the cell viability is >95%, use DMEM / F12 with pH 6.5-6.8 containing 10% FBS to culture at 37°C, 5% CO 2 The primary culture was carried out under saturated humidity, half of the medium was changed for the first time after 5 days for the P0 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com