Tolterodine fumarate oral solid preparation and preparation method thereof
A solid preparation, fumaric acid technology, applied in pharmaceutical formulations, urinary system diseases, drug combinations, etc., to achieve the effect of solving the content uniformity of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of tablet
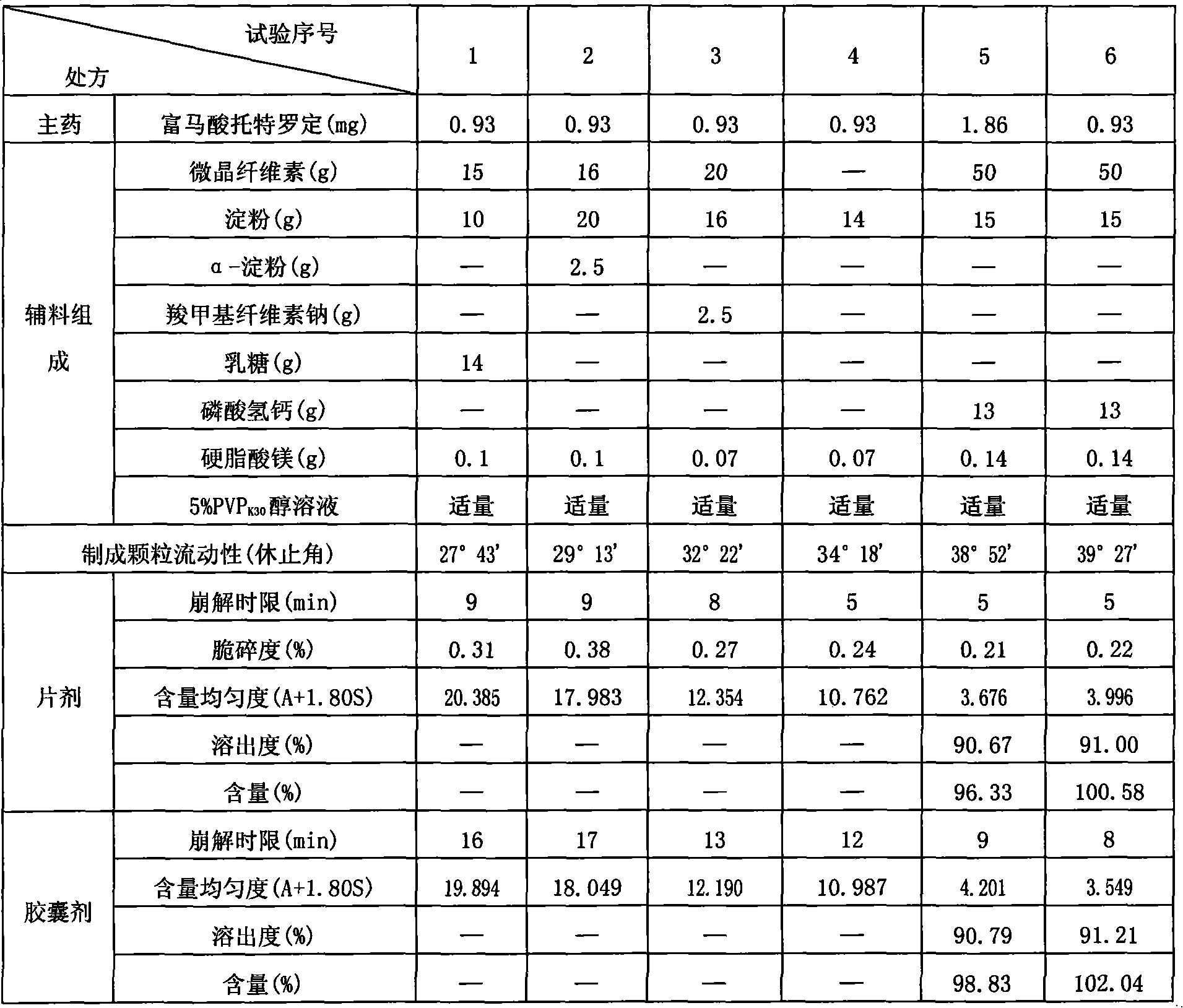
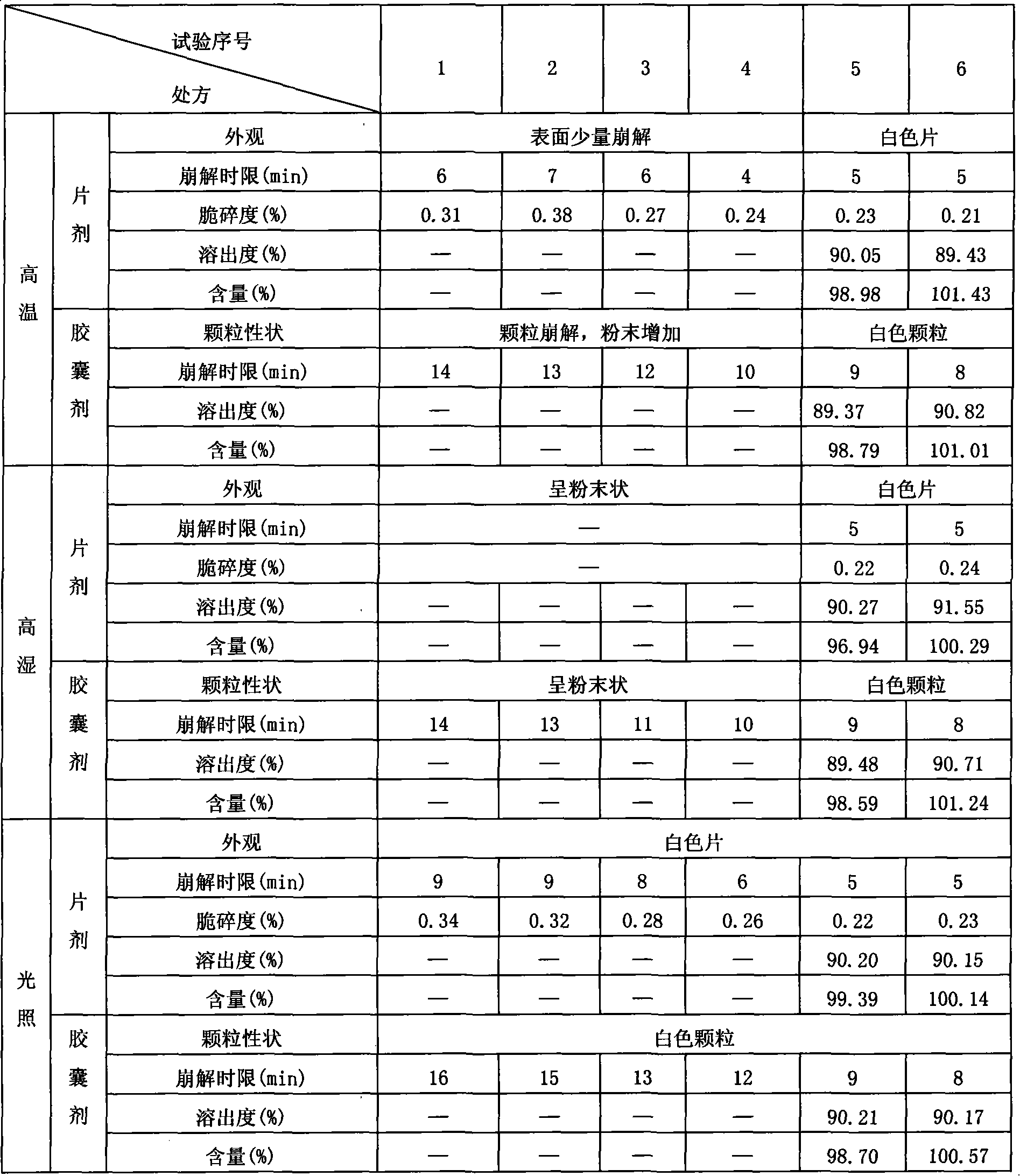
[0023] Take by weighing 0.93g of tolterodine fumarate and 25g of microcrystalline cellulose and grind together through a 100-mesh sieve, mix 50g of starch with the remaining 25g of microcrystalline cellulose and 15g of calcium hydrogen phosphate, and pass through a 100-mesh sieve , the co-researched product and the mixture are mixed in equal amounts and passed through an 80-mesh sieve, stirred evenly, and 5% povidone K is added 30 Ethanol liquid, made into soft material, granulated with a 20-mesh sieve, dried in an oven at 60°C for 2 hours, then granulated with a 20-mesh sieve, plus 0.14g of magnesium stearate as an auxiliary material, mixed to obtain granules, and pressed into tablets , that is.
Embodiment 2
[0024] Embodiment 2: the preparation of capsule
[0025] Take by weighing 1.86g of tolterodine fumarate and 60g of microcrystalline cellulose and grind together through a 100-mesh sieve, mix 30g of starch with the remaining 30g of microcrystalline cellulose and 40g of calcium hydrogen phosphate, and pass through an 80-mesh sieve , the co-research product and the mixture are mixed in equal amounts and passed through a 60-mesh sieve, stirred evenly, and 1% hydroxyethyl methylcellulose ethanol solution is added to make a soft material, granulated with a 12-mesh sieve, and dried in an oven at 80°C After 1 hour, granulate with a 12-mesh sieve, add 0.07 g of talcum powder as an auxiliary material, mix well to obtain granules, put them into empty capsule shells, and obtain.
Embodiment 3
[0026] Embodiment 3: the preparation of tablet
[0027] The tolterodine fumarate 0.93g and 60g microcrystalline cellulose that take by weighing recipe quantity grind together 60 mesh sieves, starch 5g and remaining 120g microcrystalline cellulose, calcium phosphate 10g are mixed, cross 100 mesh sieves, The co-researched product and the mixture were mixed in equal amounts through a 100-mesh sieve, stirred evenly, added 8% methylcellulose aqueous solution to make a soft material, granulated with a 40-mesh sieve, dried in an oven at 40°C for 4 hours, and then used Sieve through a 40-mesh sieve, add 0.2 g of stearic acid as an auxiliary material, mix well to obtain granules, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com