Method for preparing Tolterodine and tartrate
A technology of tolterodine and tartrate, which is applied in the field of compounds, can solve the problems of harsh reaction conditions, long synthetic process steps in industrial production, and few preparation steps, and achieve the effects of mild reaction conditions, reduced production costs, and increased yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
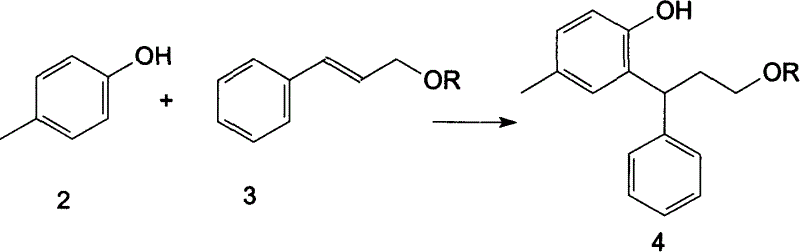
[0032] Embodiment 1: 3-(2-hydroxyl-5-methylphenyl)-3-phenylpropylene 3, the preparation method of 5-dinitrobenzoate (4)
[0033] In a four-neck flask, add p-cresol 2 (119g, 1.1mol), trans-cinnamyl alcohol 3,5-dinitrobenzoate (344g, 1.0mol), 150mL of concentrated sulfuric acid, and heat up to 135°C- 138°C, stirred and reacted for 8 hours, cooled, adjusted the pH to neutral with 10% sodium hydroxide, extracted with ethyl acetate 80mL×2, washed the organic phase with water, washed with 5% aqueous potassium carbonate solution, and anhydrous sulfuric acid Sodium dry. After filtration, the solvent was evaporated under reduced pressure, and the residue was recrystallized with 800 mL of isopropanol to obtain 3-(2-hydroxyl-5-methylphenyl)-3-phenylpropylene 3,5-dinitrobenzene Formate (366 g, 82.8%).
Embodiment 2
[0034] Embodiment 2: the preparation method of 3-(2-hydroxyl-5-methylphenyl)-3-phenylpropylene methanesulfonate (4)
[0035] In a four-neck flask, add p-cresol 2 (119g, 1.1mol), trans-cinnamyl trifluoromethanesulfonyl ester (165g, 1.0mol), 150mL of concentrated sulfuric acid, raise the temperature to 123°C-126°C, and stir the reaction After 16 hours, cool to room temperature, adjust the pH to neutral with 10% aqueous sodium hydroxide solution, extract with 800 mL×2 ethyl acetate, wash the organic phase with water, wash with 5% potassium carbonate, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was recrystallized with 800 mL of isopropanol to obtain -(2-hydroxyl-5-methylphenyl)-3-phenylpropylene methanesulfonate (156.8 g, 84.5 %).
Embodiment 3
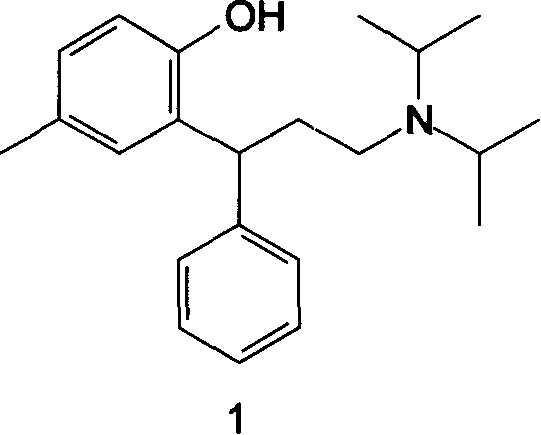
[0036] Example 3: Preparation of N, N-diisopropyl-3-(2-hydroxyl-5-methylphenyl)-3-phenylpropylamine 1
[0037] The product obtained in Example 1 (371 g, 0.84 mol) was dissolved in 1500 mL of toluene, mixed with 1500 g of diisopropylamine, refluxed and stirred for 38 hours, the solvent was evaporated, and the residue was added with 10% aqueous sodium hydroxide solution to adjust the pH 14, extracted with ethyl acetate, washed with water, decolorized, filtered, evaporated to remove ethyl acetate, and the oily product was N, N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3- Phenylpropylamine 1 (146 g, 68.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com