Application of mussaenda erythrophylla glucoside U in preparation of antiacetyl-choline medicine
A technology of euphylloside and acetylcholine, which is applied in the direction of drug combination, anti-toxic agent, pharmaceutical formula, etc., and can solve the problems such as no registration of euphylloside U preparation and no anti-acetylcholine effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 goldenrodine U (hereinafter referred to as saponin U)
[0021] Add 5kg of Jade Leaf Golden Flower coarse powder, add 50L of 60% ethanol and extract under reflux for 3 times, each time for 2 hours, combine the extracts, concentrate in a vacuum until dry to obtain a crude extract, add water to dissolve, then add an equal amount of water-saturated n-butanol to extract more Once, the organic layer was collected and concentrated in vacuo to obtain an extract-like substance. Then, the extract was subjected to macroporous resin column chromatography, followed by eluting with distilled water, 20%, 40%, 60%, and 95% ethanol solution, collecting 60%-95% ethanol eluate, recovering ethanol, and evaporating to dryness. That is, 27g of total saponin crude product was obtained. The crude total saponins were dissolved in methanol, separated by HPLC preparative chromatography, and monitored online by an ultraviolet detector to collect the target componen...
Embodiment 2
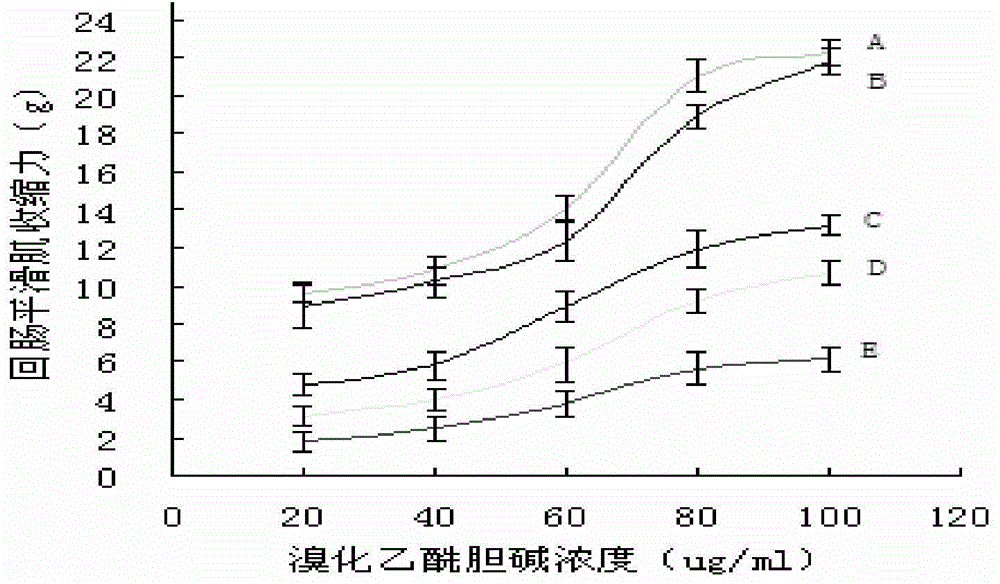
[0025] Example 2 Effect test of saponin U on contractility of isolated guinea pig ileum smooth muscle induced by acetylcholine bromide
[0026] Qualified guinea pigs were taken and killed by clubbing. A section of ileum near the cecum was quickly taken and placed in Krebs' solution to separate the longitudinal smooth muscle of the ileum. Immediately add 19.5mL of Krebs' solution to the bath, maintain a constant temperature of 37°C, pass in a mixed gas (5% carbon dioxide, 95% oxygen, the same below), draw electrical signals from the tension sensor to the biological function experiment system, adjust the tension of the silk thread, and make the muscle bundle Load 1 gram, record muscle bundle relaxation and contraction, after 30 minutes of muscle bundle relaxation and contraction stabilization, record a section of muscle contraction force curve, after that, do the following dosing experiment. The same ileal muscle bundle can only be used for one test and will not be used repeated...
Embodiment 3
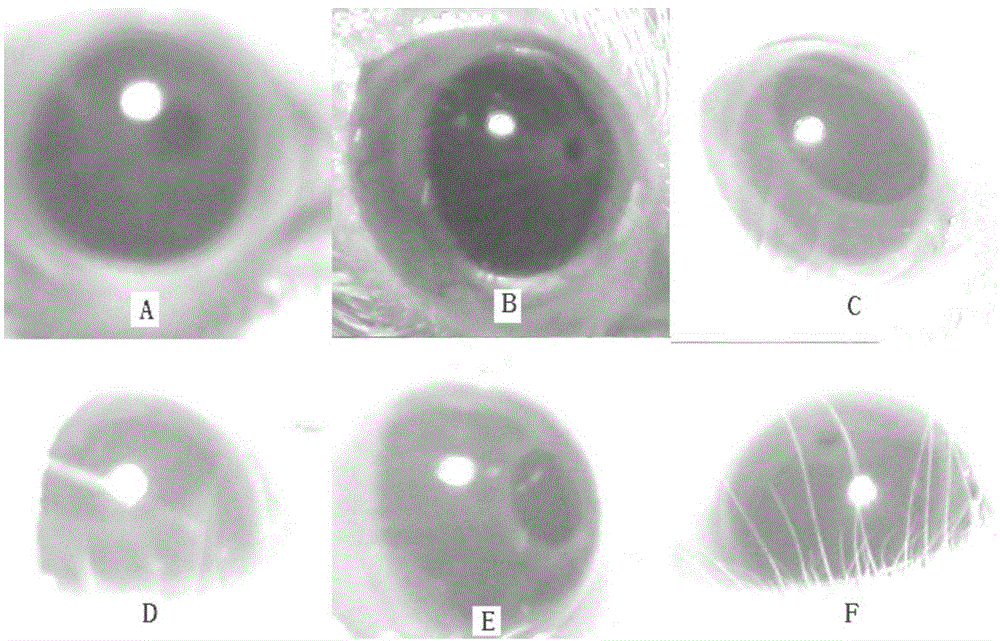
[0030] Example 3 Effect Test of Saponin U on the Propelling Percentage of Small Intestine Carbon End in M Cholinergic Nervous Excitability Model Mice
[0031] Take 60 fasting mice and randomly divide them into normal group, model group, positive drug (atropine sulfate) group and three administration test groups, 10 in each group. Except for the normal group, the other groups were treated with 10ml.kg -1 Injection volume, intraperitoneal injection concentration is 2.0mg.ml -1 Pilocarpine liquid medicine, dosage 20.0mg.kg -1 . 15 minutes after the injection, the model group was injected with distilled water 10ml.kg into the tail vein -1 ; Positive drug group tail vein injection concentration is 0.025mg.ml -1 Atropine sulfate solution 10ml.kg -1 , dose 2.5mg.kg -1 ; The concentration of tail vein injection in the three administration test groups was 1.0, 0.5, 0.25mg.ml -1 Saponin U liquid 10ml.kg -1 , the doses were 10.0, 5.0, 2.5mg.kg -1 ; The normal group was not adm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com