Levetiracetam sustained-release tablets and preparation method thereof
A slow-release tablet and slow-release technology, applied in the field of medicine, can solve problems such as adverse reactions and difficulty in controlling patients' seizures, and achieve the effects of stable blood drug concentration, good development prospects, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] Below enumerate several representative in specific embodiment, illustrate the specific implementation mode of this method.
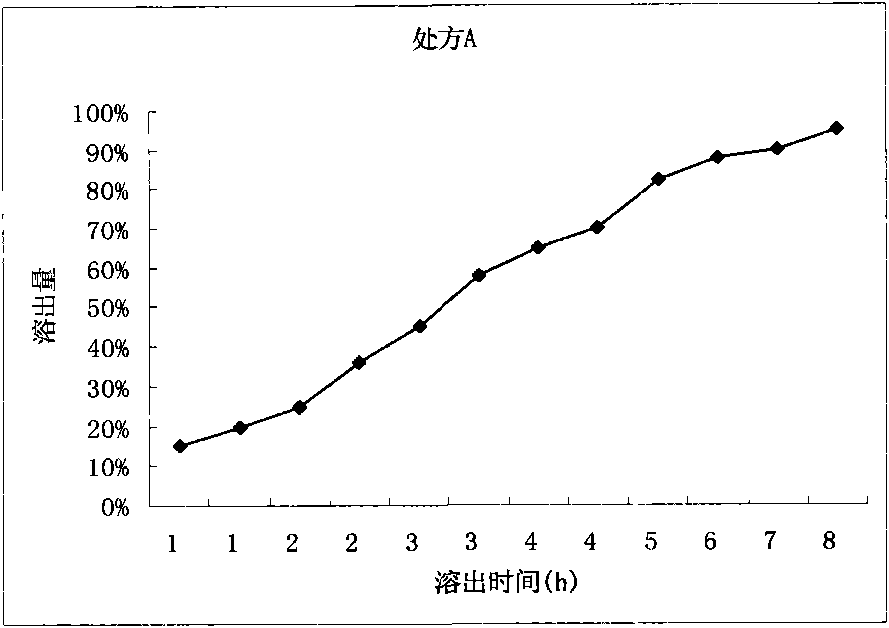
[0022] Prescription A:
[0023] Levetiracetam 300g
[0024] HPMC-4M 15g
[0025] Lactose 90g
[0026] 10% PVP 95% ethanol solution 120g
[0027] Glyceryl Behenate 3g
[0028] Preparation process: Pass Levetiracetam and HPMC-4M through 80 mesh sieve, weigh the prescribed amount of Levetiracetam, HPMC-4M and lactose, mix evenly, add 10% PVP 95% ethanol solution in an appropriate amount, granulate, Dry at 60°C, sieve through a 20-mesh sieve, and granulate. Add the prescribed amount of behenic acid glyceride to the dry granules, mix well, and press into tablets.
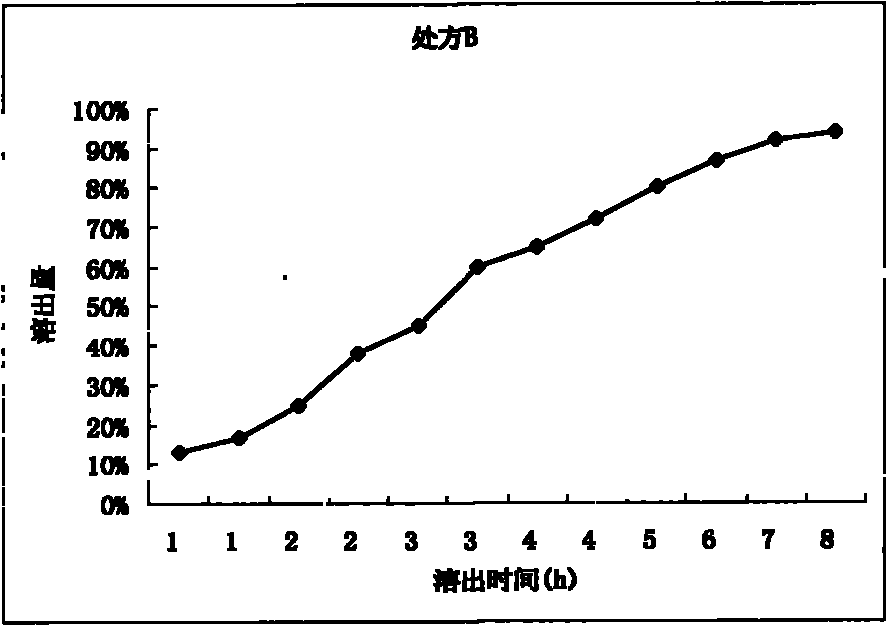
[0029] Prescription B:
[0030] Levetiracetam 300g
[0031] HPMC-4M 120g
[0032] Lactose 90g
[0033] 8% PVP 95% ethanol solution 100g
[0034] Glyceryl Behenate 3g
[0035] The preparation process is the same as prescription A.
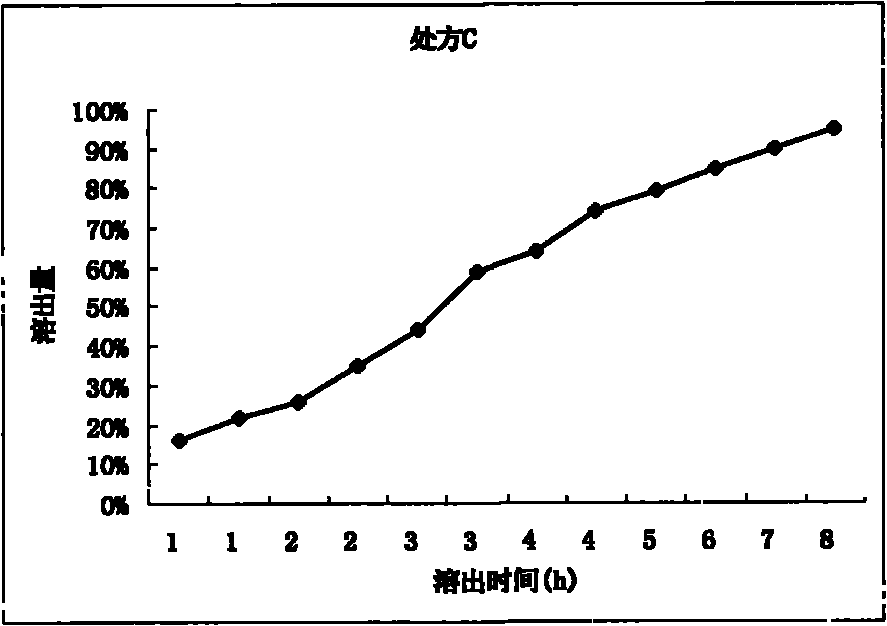
[0036] Prescription C:
[0037] Levetiracetam 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com