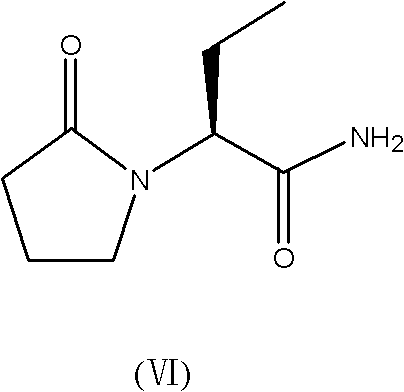
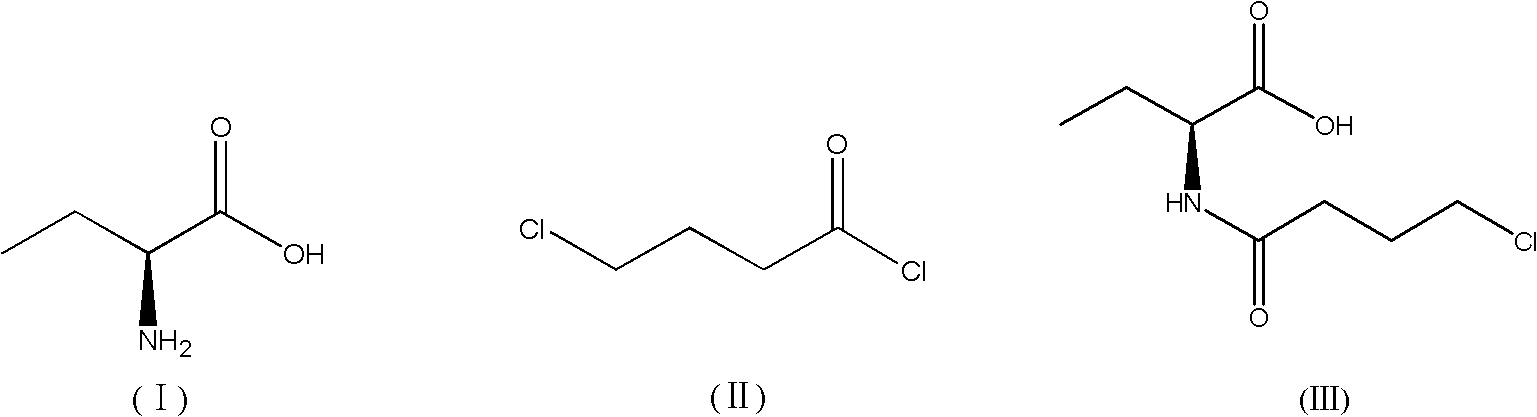
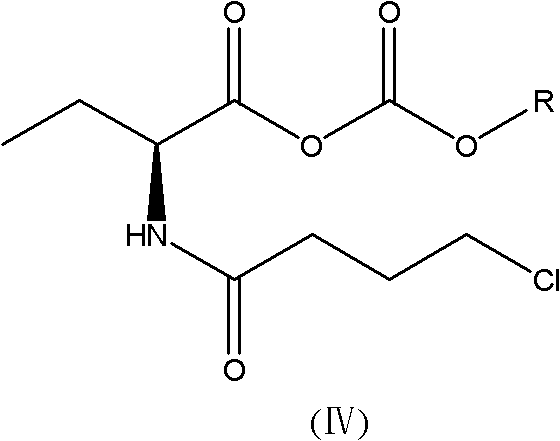
Synthesis method of levetiracetam
A synthetic method and selected technology, applied in the field of drug synthesis, can solve the problems of low overall yield, high toxicity and corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Alkylation reaction: add (S)-2-aminobutyric acid 1.00mol (103g), 500mL water and 87g alkaline reagent NaHCO into a 1000mL three-neck flask 3 , then lower the temperature to 0°C under stirring, control the temperature within the range of 0°C to 5°C, add 1.05mol (148g) of 4-chlorobutyryl chloride dropwise, and continue to keep the temperature at 0°C to 5°C The alkylation reaction was carried out for 3 hours. After the alkylation reaction was finished, post-treatment was carried out. The post-treatment was as follows: add 200 mL of extractant dichloromethane to the reaction solution, stir for 20 min, let stand for 30 min, separate layers, collect the organic phase, Then add 8 g of desiccant anhydrous sodium sulfate to the organic phase, stir and dry for 30 minutes, filter, collect the filtrate, carry out vacuum distillation to the obtained filtrate, control the temperature of the vacuum distillation to be 20° C. to 60° C., and carry out the vacuum distillation to No liquid...
Embodiment 2
[0064] Alkylation reaction: Add 1.00mol (103g) of (S)-2-aminobutyric acid, 500mL of water and 1.20mol (106g) of alkaline reagent Na to a 1000mL three-necked flask 2 CO 3 , then cooled down to -5°C under stirring, controlled the temperature in the range of -5°C to 0°C, added 1.20mol (169g) of 4-chlorobutyryl chloride dropwise, and continued to keep the temperature at -5°C to 0°C Under the condition of ℃, the alkylation reaction was carried out for 4 hours. After the alkylation reaction was completed, post-treatment was carried out. The post-treatment was as follows: add 200 mL of extractant ethyl acetate to the reaction solution, stir for 20 minutes, let stand for 30 minutes, separate layers, and collect Organic phase, then add 10 g of desiccant anhydrous sodium sulfate to the organic phase, stir and dry for 30 minutes, filter, collect the filtrate, carry out vacuum distillation to the obtained filtrate, control the temperature of vacuum distillation to be 40 ℃ ~ 50 ℃, reduce ...
Embodiment 3
[0069] Alkylation reaction: Add 1.00mol (103g) of (S)-2-aminobutyric acid, 500mL of water and 1.00mol (138g) of alkaline reagent K into a 1000mL three-necked flask 2 CO 3, then lower the temperature to 5°C under stirring, control the temperature within the range of 5°C to 10°C, add 1.15mol (162g) of 4-chlorobutyryl chloride dropwise, and continue to keep the temperature at 5°C to 10°C The alkylation reaction was carried out for 1 hour. After the alkylation reaction was completed, post-treatment was carried out. The post-treatment was as follows: add 200 mL of extractant 2-methyltetrahydrofuran to the reaction solution, stir for 20 min, let stand for 30 min, separate layers, and collect organic phase, then add 10 g of desiccant anhydrous sodium sulfate to the organic phase, stir and dry for 30 minutes, filter, collect the filtrate, carry out vacuum distillation on the obtained filtrate, control the temperature of the vacuum distillation to 30°C to 50°C, and depressurize Distil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com