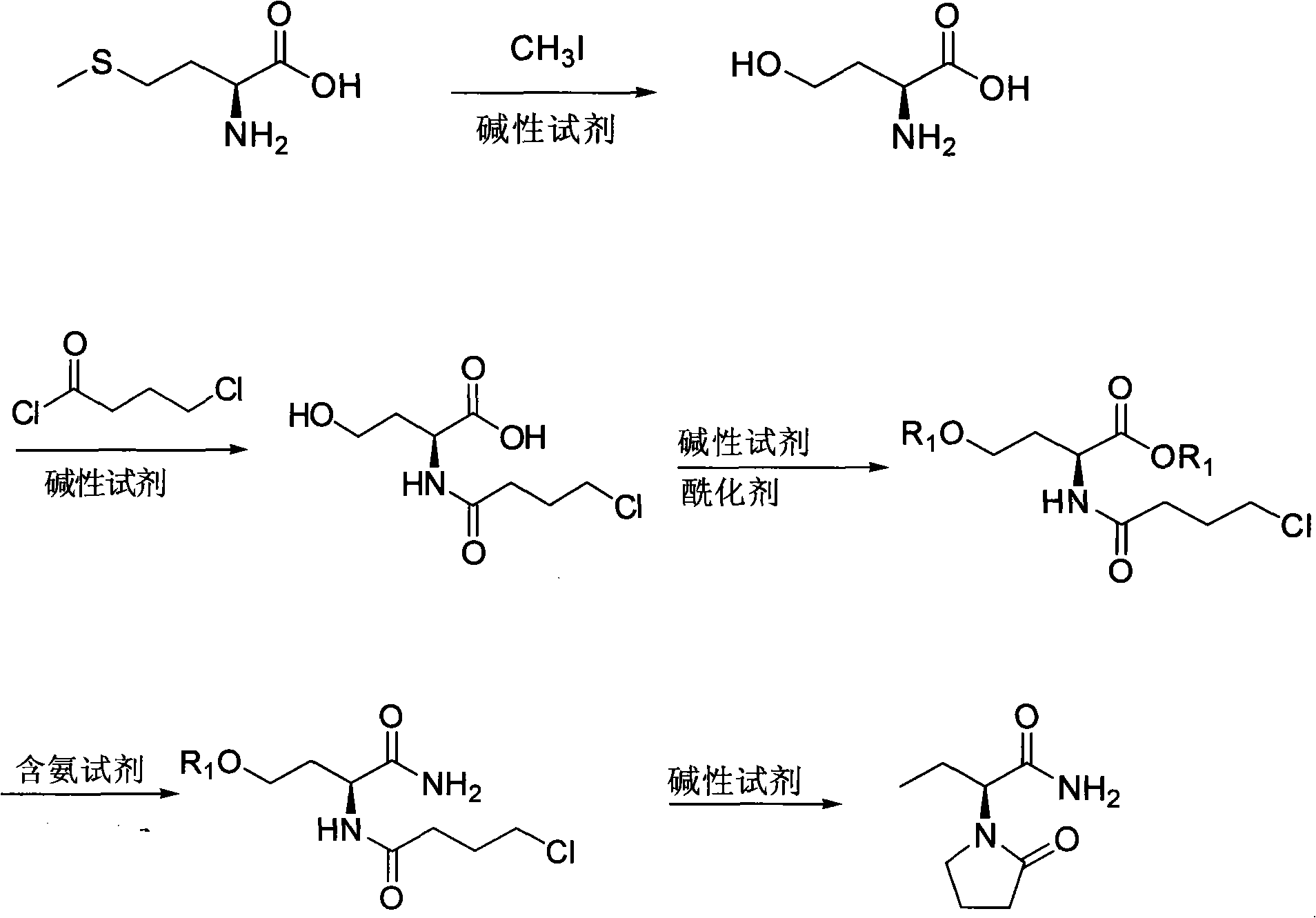
Method for preparing Levetiracetam
A compound and basic reagent technology, applied in the field of drug preparation, can solve problems such as unsatisfactory optical purity of reaction products, low total product yield, complicated reaction steps, etc., and achieve the required conditions for the reaction and the reaction process is simple and inexpensive Low cost and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Compound (II) is the synthesis of (S)-2-amino-4-hydroxybutyric acid:
[0036] Dissolve 7.5g of L-methionine (0.05mol) in 200ml of water, add 10ml (0.16mol) of methyl iodide dropwise, seal and stir at 30°C for 24 hours, concentrate under reduced pressure to 100ml to remove excess methyl iodide, add 5.00g of KHCO 3 (0.05mol) heated to reflux for 10h, vacuum-dried at 60°C, added 100ml of methanol and 1ml of water to dissolve, added dropwise concentrated hydrochloric acid to adjust the pH value to 5-6, filtered while hot, concentrated to 30ml, frozen and crystallized, suction filtered, 70 After drying at °C for 5 hours, 4.55 g of white crystals of (S)-2-amino-4-hydroxybutyric acid were obtained, with a yield of 70%.
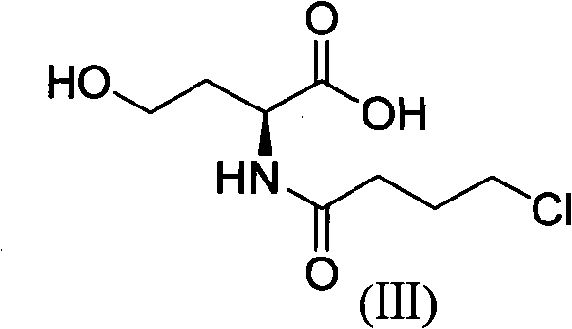
[0037] (2) Compound (III) is the synthesis of (S)-2-(4-chlorobutyrylamide)-4-hydroxybutyric acid:
[0038] Take 3.23g (0.027mol) of (S)-2-amino-4-hydroxybutyric acid synthesized in step (1), add 9.07g (0.108mol) of sodium bicarbonate and mix well, add to ...
Embodiment 2
[0044] (1) Compound (II) is the synthesis of (S)-2-amino-4-hydroxybutyric acid:
[0045] Dissolve 7.5g of L-methionine (0.05mol) in 200ml of water, add 8ml (0.13mol) of methyl iodide dropwise, seal and stir at 30°C for 24 hours, concentrate under reduced pressure to 100ml to remove excess methyl iodide, and add 2.00g of sodium hydroxide (0.05mol) heated to reflux for 5h, vacuum-dried at 50°C, added 100ml of ethanol and 1ml of water to dissolve, added dropwise concentrated hydrochloric acid to adjust the pH value to 5-6, filtered while hot, concentrated to 30ml, frozen and crystallized, suction filtered, 70 After drying at °C for 5 hours, 5.00 g of white crystals of (S)-2-amino-4-hydroxybutyric acid were obtained, with a yield of 76.9%.
[0046] (2) Compound (III) is the synthesis of (S)-2-(4-chlorobutyrylamide)-4-hydroxybutyric acid:
[0047] Take 3.23g (0.027mol) of (S)-2-amino-4-hydroxybutyric acid synthesized in step (1), add 11.45g (0.108mol) of sodium carbonate and mix w...
Embodiment 3
[0053] (1) Compound (II) is the synthesis of (S)-2-amino-4-hydroxybutyric acid:
[0054] Dissolve 7.5g of L-methionine (0.05mol) in 200ml of water, add 12.5ml (0.20mol) of methyl iodide dropwise, seal and stir at 30°C for 24 hours, concentrate under reduced pressure to 100ml to remove excess methyl iodide, add 5.30g of sodium carbonate (0.05mol) heated to reflux for 11h, vacuum-dried at 50°C, added 100ml of acetone and 1ml of water to dissolve, added dropwise concentrated hydrochloric acid to adjust the pH value to 5-6, filtered while hot, concentrated to 30ml, frozen and crystallized, suction filtered, 60 After drying at °C for 5 hours, 4.12 g of white crystals of (S)-2-amino-4-hydroxybutyric acid were obtained, with a yield of 63%.
[0055] (2) Compound (III) is the synthesis of (S)-2-(4-chlorobutyrylamide)-4-hydroxybutyric acid:
[0056] Take 3.23g (0.027mol) of (S)-2-amino-4-hydroxybutyric acid synthesized in step (1), add 2.16g (0.054mol) of sodium hydroxide and mix well...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com