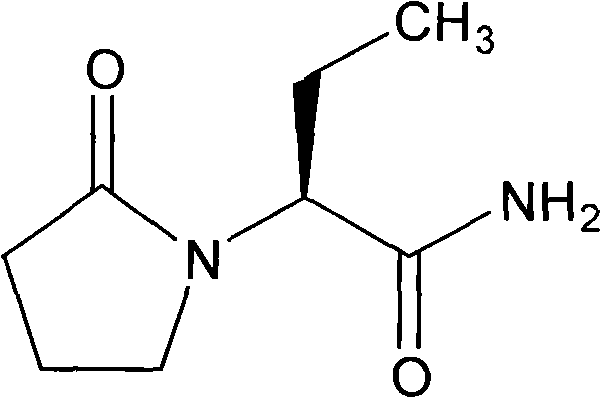
Levetiracetam tablet and preparation method thereof
A tablet and adhesive technology, applied in the field of levetiracetam tablets and its preparation, can solve the problems that the process is not conducive to cost reduction, many influencing factors, complicated process, etc., and achieve good dissolution kinetic stability , Improving the physical properties and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] According to the levetiracetam tablet prescription of table 1, prepare levetiracetam tablet according to the following steps:
[0091] a) passing levetiracetam through a 20 mesh sieve,
[0092] b) Mix the sieved levetiracetam with partially pregelatinized starch, copovidone, croscarmellose sodium, and micropowder silica gel,
[0093] c) process the mixed material obtained in step b through a 20 mesh sieve,
[0094] d) adding magnesium stearate to the sieved material obtained in step c, mixing,
[0095] e) compressing the mixture obtained in step d to prepare a tablet,
[0096] f) Coating the resulting tablet with a coating material.
[0097] Table 1
[0098]
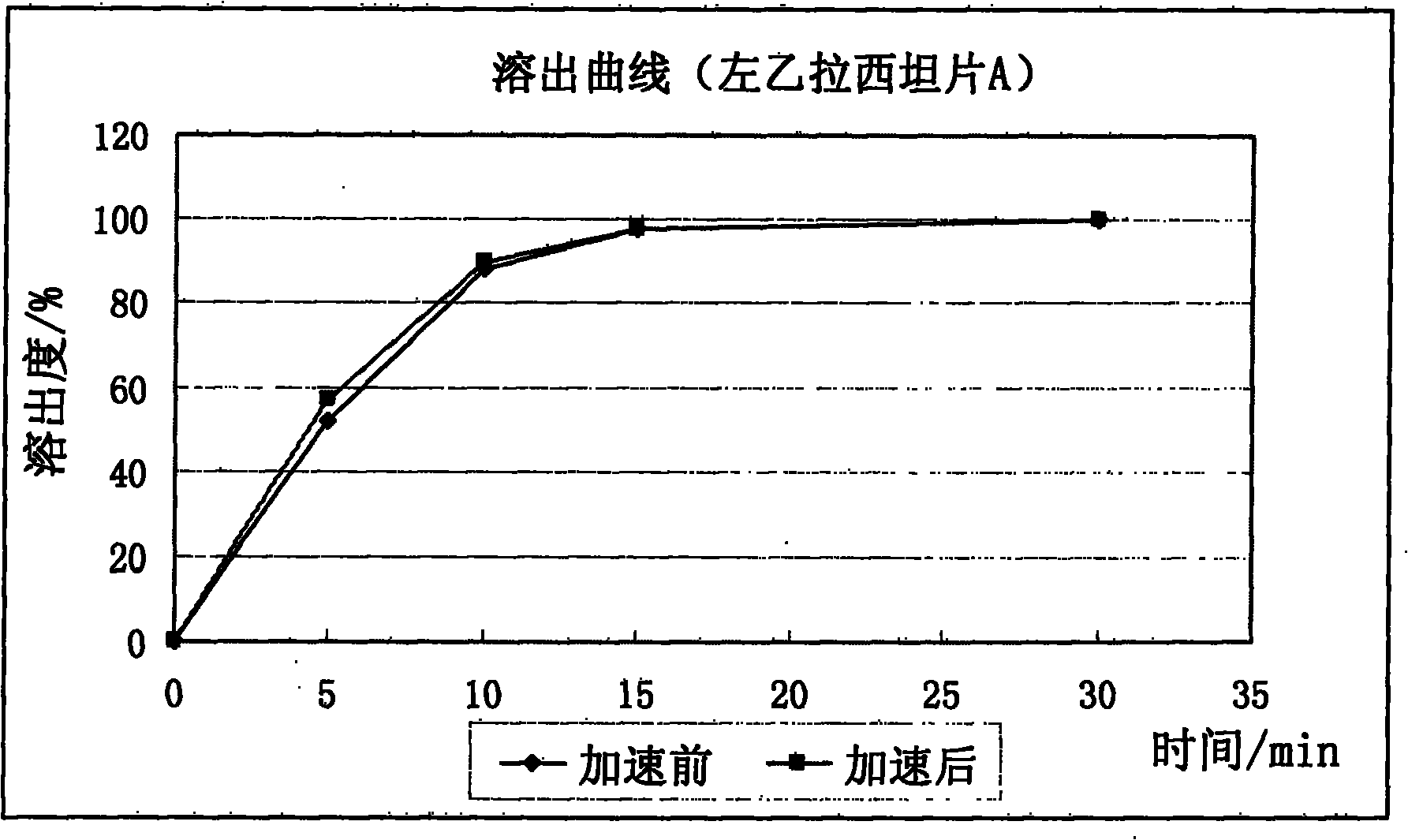
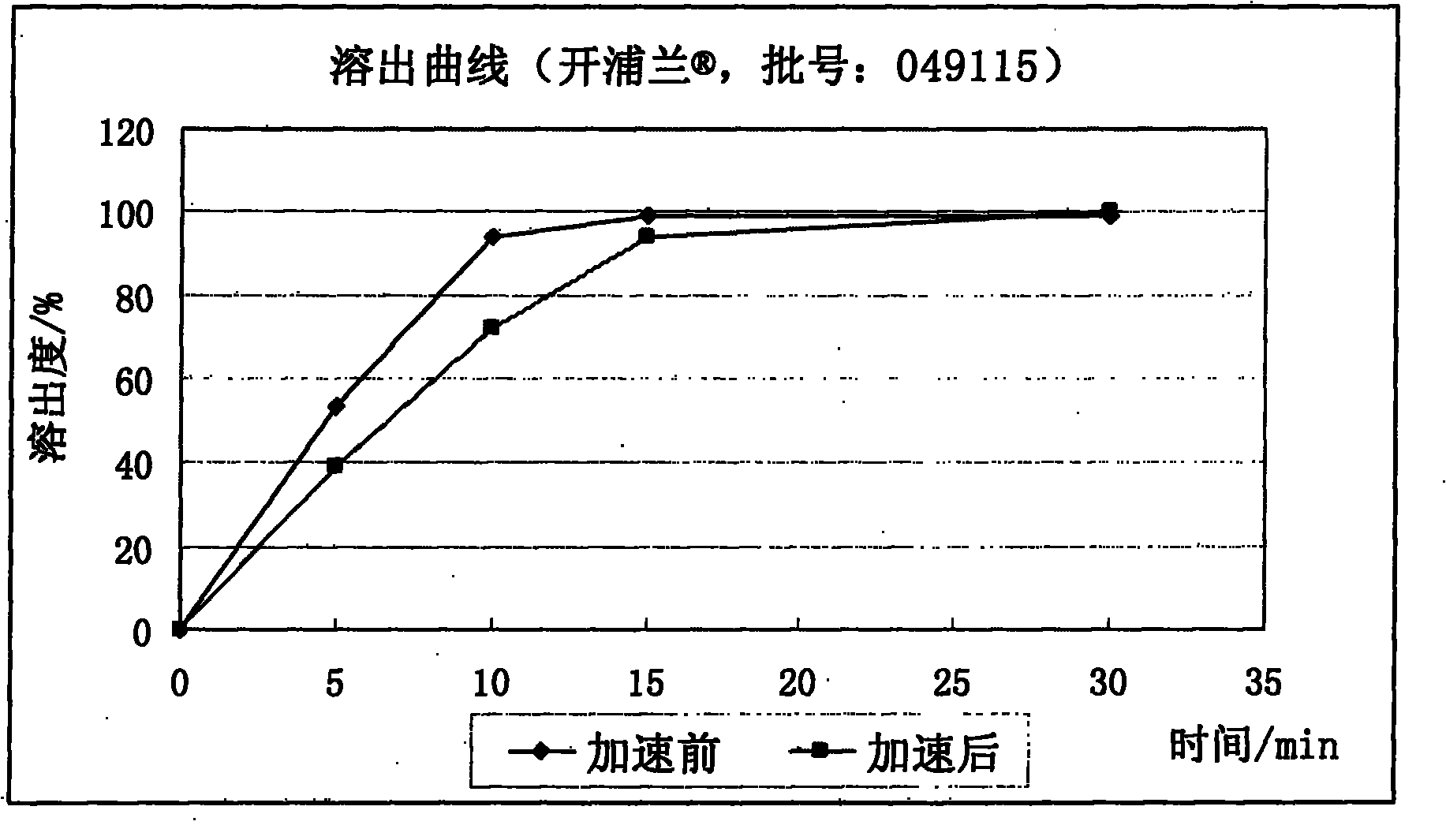
[0099] With the levetiracetam sheet A in embodiment 1, and the 250mg specification tablet of prior art product (the Kaiplan of UCB company) Batch number: 049115), after being packed in the same aluminum foil bag, placed under the condition of 60°C / 75% relative humidity, after accelerating for 2 weeks, acc...
Embodiment 2
[0107] According to the levetiracetam tablet prescription of table 3, prepare levetiracetam tablet according to the following steps:
[0108] a) passing levetiracetam through a 20 mesh sieve,
[0109] b) Mix the sieved levetiracetam with microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium and micropowder silica gel evenly,
[0110] c) process the mixed material obtained in step b through a 20 mesh sieve,
[0111] d) adding magnesium stearate to the sieved material obtained in step c, mixing,
[0112] e) compressing the mixture obtained in step d to prepare a tablet,
[0113] f) Coating the resulting tablet with a coating material.
[0114] table 3
[0115]
Embodiment 3
[0117] According to the levetiracetam tablet prescription of table 4, prepare levetiracetam tablet according to the following steps:
[0118] a) passing levetiracetam through a 20 mesh sieve,
[0119] b) Mix the sieved levetiracetam with microcrystalline cellulose, copovidone, croscarmellose sodium and micropowder silica gel evenly,
[0120] c) process the mixed material obtained in step b through a 20 mesh sieve,
[0121] d) adding magnesium stearate to the sieved material obtained in step c, mixing,
[0122] e) compressing the mixture obtained in step d to prepare a tablet,
[0123] f) Coating the resulting tablet with a coating material.
[0124] Table 4
[0125]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com