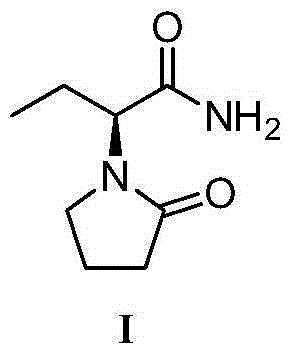
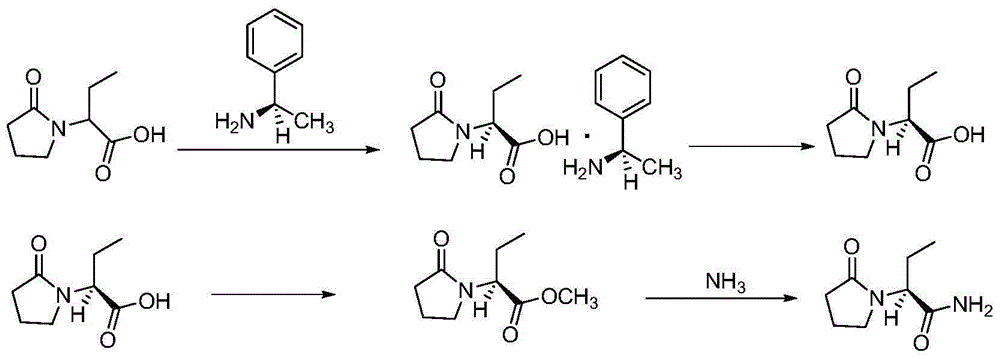
Preparation method of levetiracetam
A technology of methyl halobutyrate and quality, applied in the field of preparation of levetiracetam, can solve the problems of low yield and low industrial application value, and achieves increased comprehensive yield, enantioselectivity and regional The effect of high selectivity and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) In a 500mL three-necked flask, add 270mL of sodium dihydrogen phosphate / sodium hydrogen phosphate buffer solution with a pH value of 6.5, racemic methyl 2-chlorobutyrate (0.2mol, 27.3g) and lipase ( Novozymes immobilized lipase, CALB-Novazym435) 2.7g, control the reaction temperature at 20-25°C, carry out enantioselective hydrolysis reaction, the reaction is complete after 24 hours, add ethyl acetate for extraction (30mL×3), collect The organic layer was spin-dried to obtain 11.5 g of (R)-methyl 2-chlorobutyrate, with a yield of 84.2%. The pH value of the aqueous layer was adjusted to neutral with 4M hydrochloric acid, and then extracted with ethyl acetate. The organic layer was collected and spin-dried to obtain 9.8 g of by-product (S)-2-chlorobutyric acid, with a yield of 80.0%.
[0044] (2) In a 1L autoclave, add the (R)-2-chlorobutyric acid methyl ester (0.073mol, 10g) obtained in step (1) and 200mL of methanol, dissolve it, cool it down to -10~0℃, pass The amm...
Embodiment 2
[0047] (1) In a 500mL three-necked flask, add 350mL of sodium dihydrogen phosphate / sodium hydrogen phosphate buffer solution with a pH value of 7.0, racemic methyl 2-chlorobutyrate (0.2mol, 27.3g) and lipase ( Novozymes immobilized lipase, CALB-Novazym435) 1.4g, control the reaction temperature at 35-40°C, carry out enantioselective hydrolysis reaction, after 36 hours the reaction is complete, add ethyl acetate for extraction (30mL×3), collect The organic layer was spin-dried to obtain 11.2 g of (R)-methyl 2-chlorobutyrate, with a yield of 82.0%. The pH value of the aqueous layer was adjusted to neutral with 4M hydrochloric acid, then extracted with ethyl acetate, the organic layer was collected, and spin-dried to obtain 9.0 g of by-product (S)-2-chlorobutyric acid, with a yield of 73.4%.
[0048] (2) In a 1L autoclave, add the (R)-2-chlorobutyric acid methyl ester (0.073mol, 10g) obtained in step (1) and 350mL of methanol, dissolve it, cool to -10~0℃, pass The ammonia gas is...
Embodiment 3
[0051] (1) In a 1L three-necked flask, add 410 mL of potassium dihydrogen phosphate / potassium hydrogen phosphate buffer solution with a pH value of 7.5, racemic methyl 2-chlorobutyrate (0.2 mol, 27.3 g) and lipase ( Novozymes immobilized lipase, CALB-Novazym435) 1.9g, control the reaction temperature at 25-30°C, carry out enantioselective hydrolysis reaction, after 48 hours the reaction is complete, add ethyl acetate for extraction (30mL×3), collect The organic layer was spin-dried to obtain 12.0 g of (R)-methyl 2-chlorobutyrate, with a yield of 87.9%. The pH value of the aqueous layer was adjusted to neutral with 4M hydrochloric acid, then extracted with ethyl acetate, the organic layer was collected, and spin-dried to obtain 10.5 g of by-product (S)-2-chlorobutyric acid, with a yield of 85.7%.
[0052] (2) In a 1L autoclave, add the (R)-2-chlorobutyric acid methyl ester (0.073mol, 10g) obtained in step (1) and 500mL of ethanol, dissolve it, cool down to -10~0℃, pass The amm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com