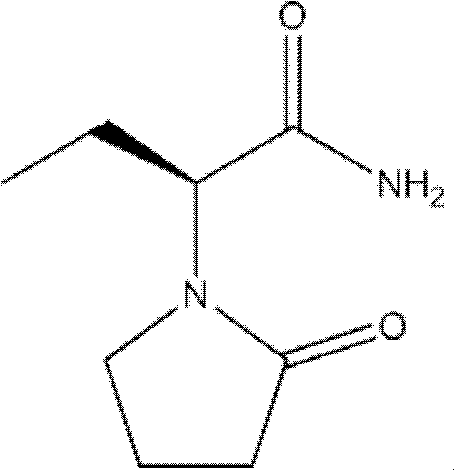
Preparation method of levetiracetam
A technology of triethylamine and inert solvent, applied in the field of preparation of antiepileptic drug levetiracetam, can solve the problems of difficult industrialized operation, expensive reagents, low total yield and the like, and achieves easy control of the operation process, optical The effect of ideal purity and lower raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
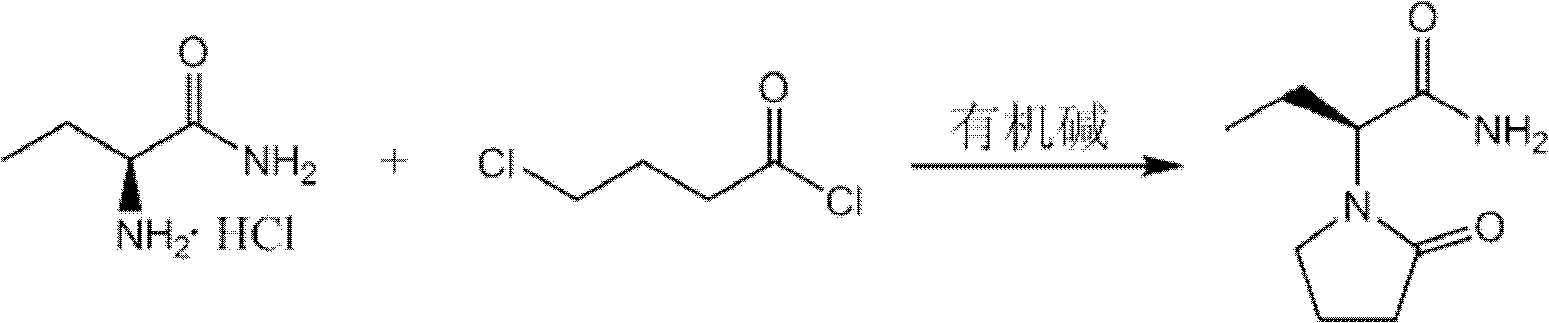
[0038] The preparation method of levetiracetam provided by the invention comprises: 1) in an inert solvent, under a suitable organic base environment, make (S)-2-aminobutyramide hydrochloride and 4-chlorobutyryl chloride directly carry out one-step reaction Make levetiracetam, its reaction formula is as shown in following reaction formula (I):
[0039]
[0040] Reaction formula (I)
[0041] Wherein, the organic base of the present invention may be trimethylamine, triethylamine, tripropylamine or diisopropylethylamine, preferably tripropylamine or diisopropylethylamine. The yield of levetiracetam obtained by the method of the invention is ideal and the optical purity is high.
[0042] According to one embodiment of the present invention, the inert solvent in the present invention may be an inert organic solvent, such as benzene, toluene, xylene, methylene chloride, 1,2-dichloroethane, chloroform, preferably chloroform. The amount of the inert solvent can be 3 to 20 times t...
Embodiment approach
[0043] According to another embodiment of the present invention, the molar ratio of (S)-2-aminobutyramide hydrochloride, 4-chlorobutyryl chloride and the preferred organic base of the present invention can be 1: (1~5): (3~15). In the method of the present invention, if the molar ratio of 4-chlorobutyryl chloride and the organic base is too high, the cost of raw materials will be increased; if the molar ratio is too low, the reaction will be incomplete, resulting in reduced product yield and purity. Preferably, the molar ratio of (S)-2-aminobutyramide hydrochloride, 4-chlorobutyryl chloride and the preferred organic base is 1:(1.1-3):(2-6).
[0044] According to another embodiment of the present invention, the reaction temperature in the present invention may be -15°C to 15°C. In the method of the present invention, if the reaction temperature is too high, the product will racemize and reduce the optical purity of the product; if the reaction temperature is too low, the reacti...
Embodiment 1
[0066] With (S)-2-aminobutyramide hydrochloride (69.3 grams, 0.5mol), chloroform (831.6 grams), tripropylamine (300.7g, 2.1mol), join in the three-necked reaction flask that cryogenic thermometer is housed, stir After 10 minutes, start to cool down after uniformity. When it drops to -5°C, start to drop 4-chlorobutyryl chloride (141g, 1mol). , keep warm at 0~5 ℃ and continue to react for 4 hours, the raw material controlled in HPLC disappears, the product purity is 95.52%, stop the reaction, remove the solid in the reaction solution by suction filtration, and the filtrate is evaporated to dryness under reduced pressure (0.09MPa) at 50 ℃ to obtain Pale yellow solid, add 600ml of ethyl acetate to reflux until clarified, cool down naturally, refreeze (-5°C) for crystallization for 5 hours, filter with suction, wash the filter cake with glacial ethyl acetate (-5°C) until white, bake at 60°C After drying, 72.25 g of levetiracetam was obtained, with a yield of 84.9%, a liquid chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com