Patents
Literature
33 results about "Serum phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical-Grade Ferric Organic Compounds, Uses Thereof and Method of Making Same
ActiveUS20080274210A1Long-term administrationHeavy metal active ingredientsOrganic active ingredientsDiseaseSerum phosphate
Owner:PANION & BF BIOTECH INC
Pharmaceutical-grade ferric organic compounds, uses thereof and method of making same
ActiveUS8093423B2Improve scalabilityImprove the preparation effectHeavy metal active ingredientsBiocideSerum phosphateSimple Organic Compounds
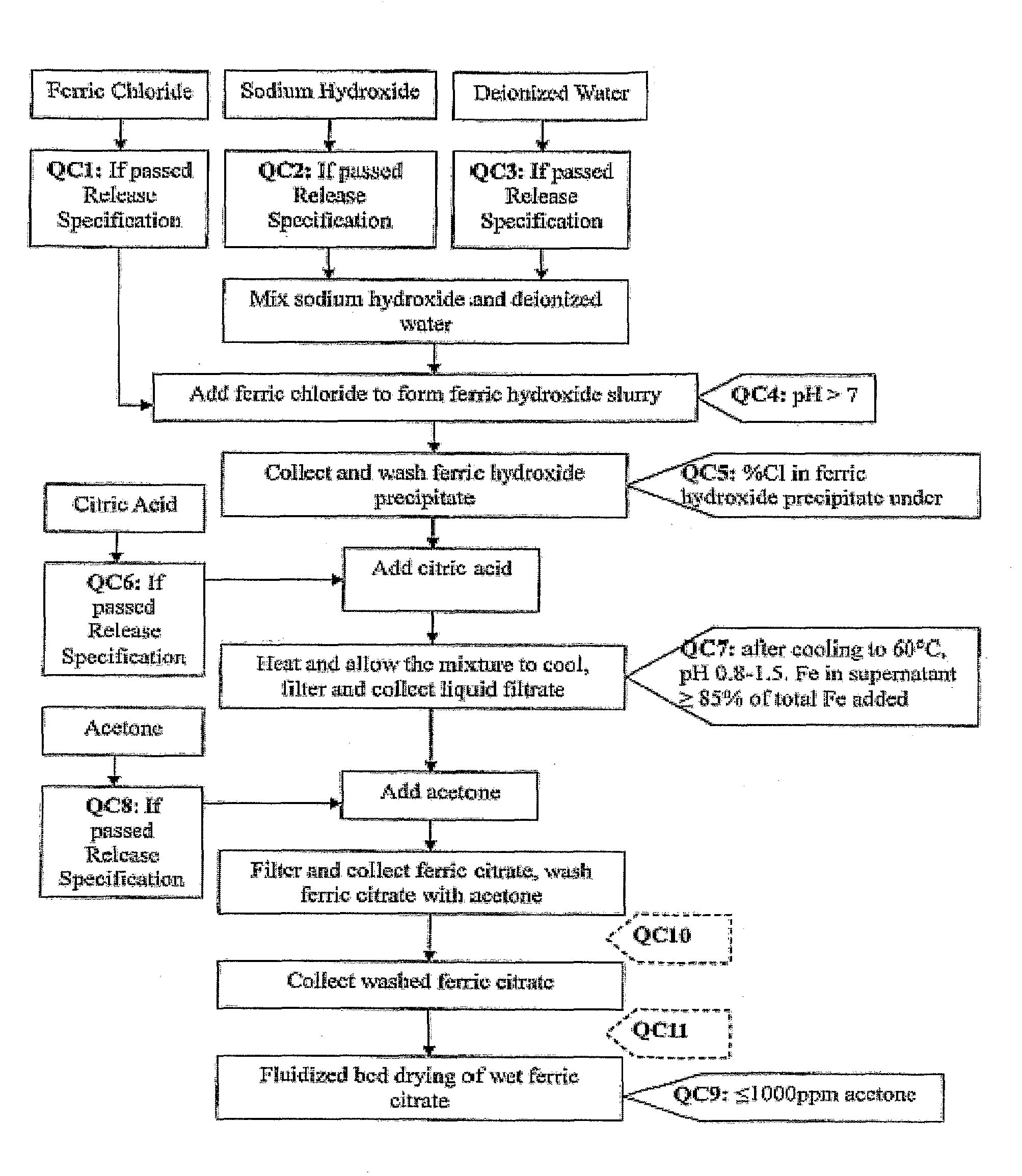
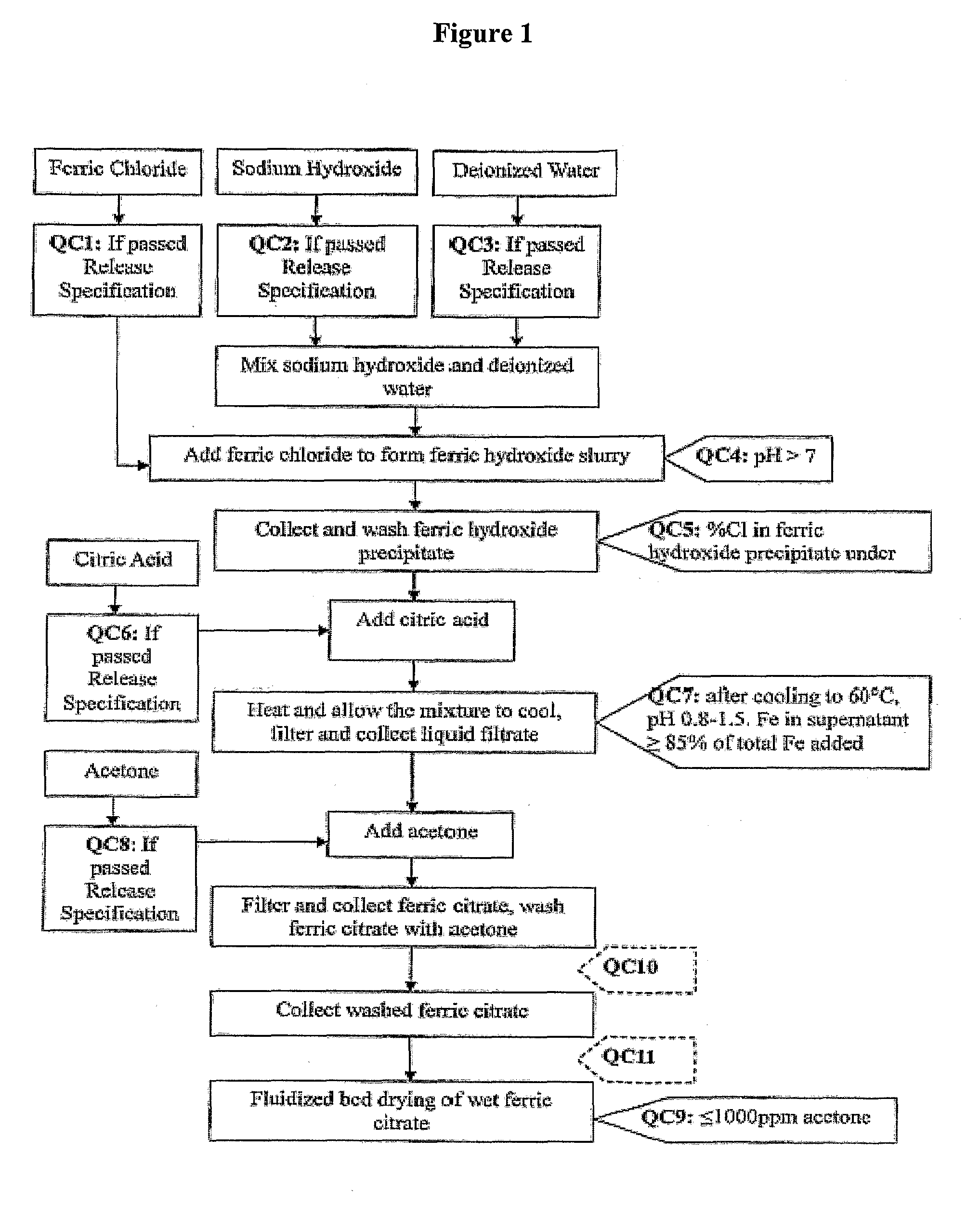
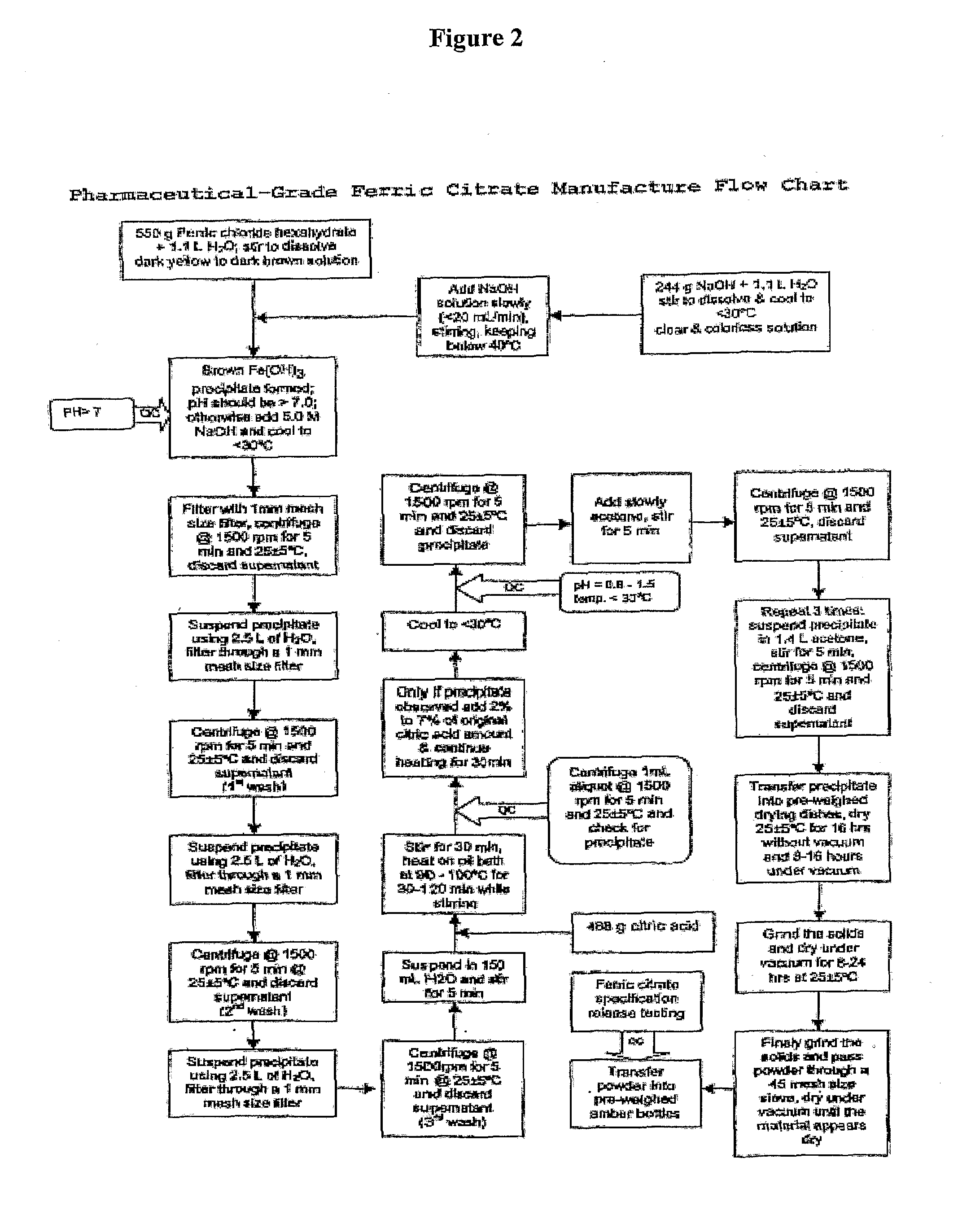
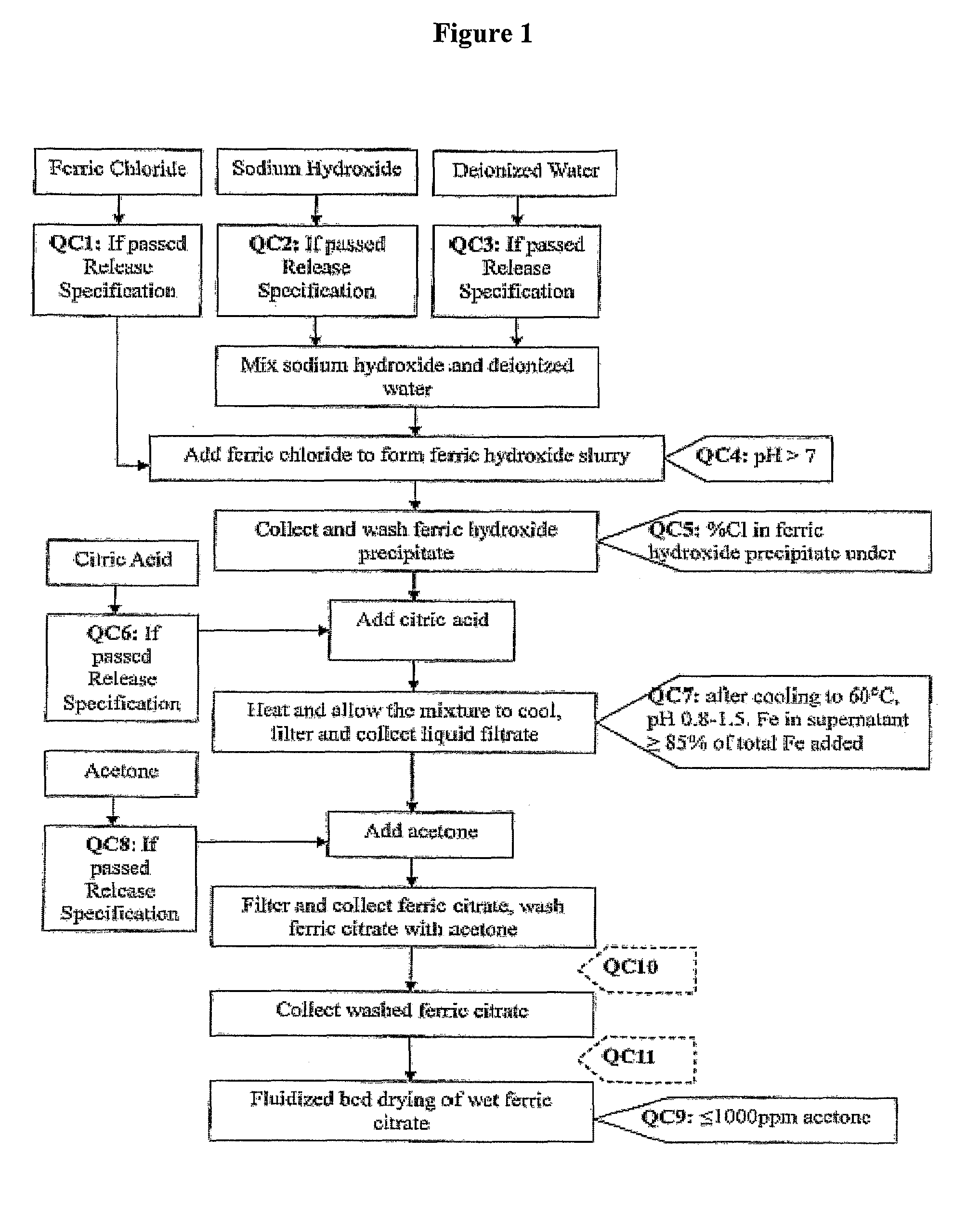
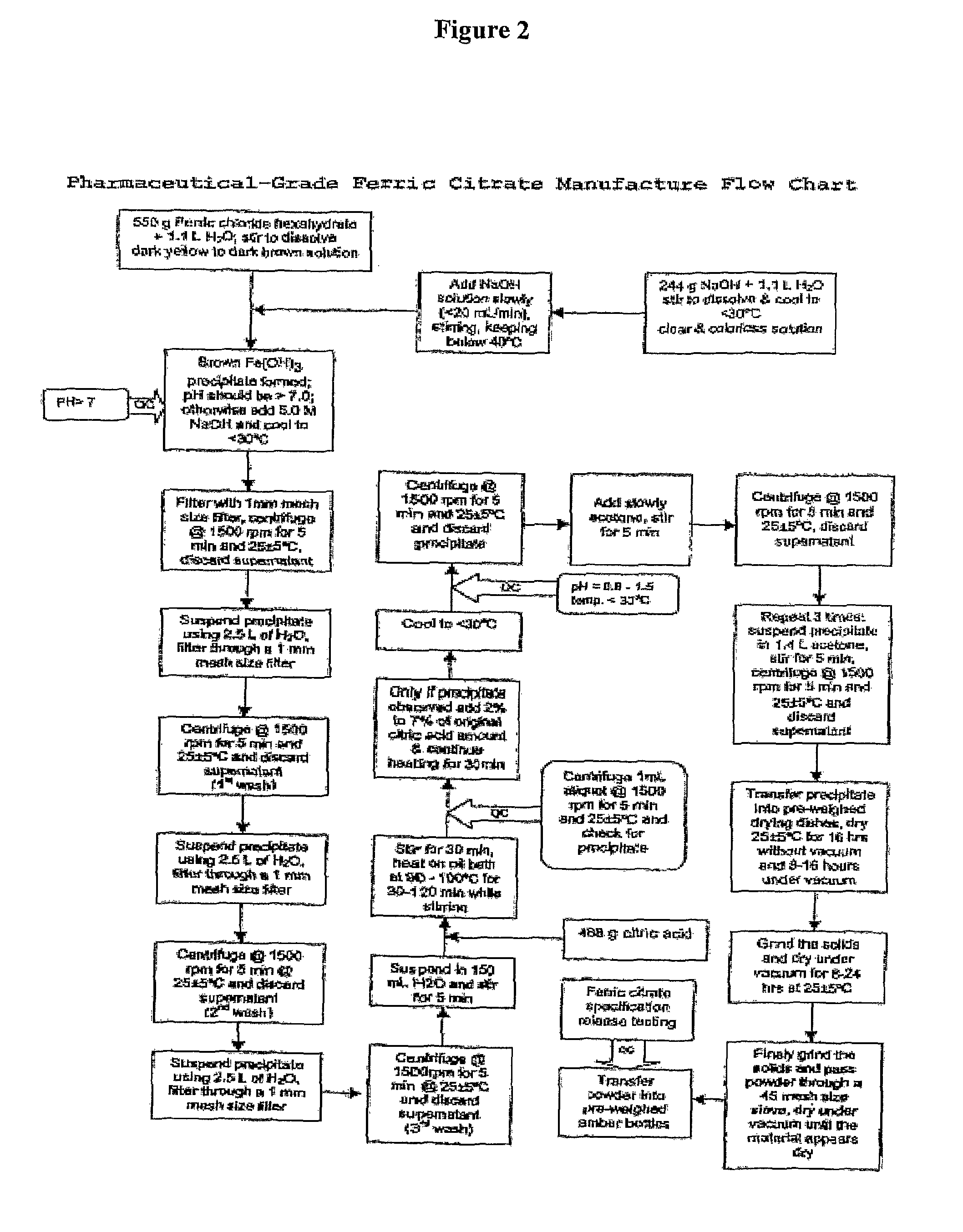
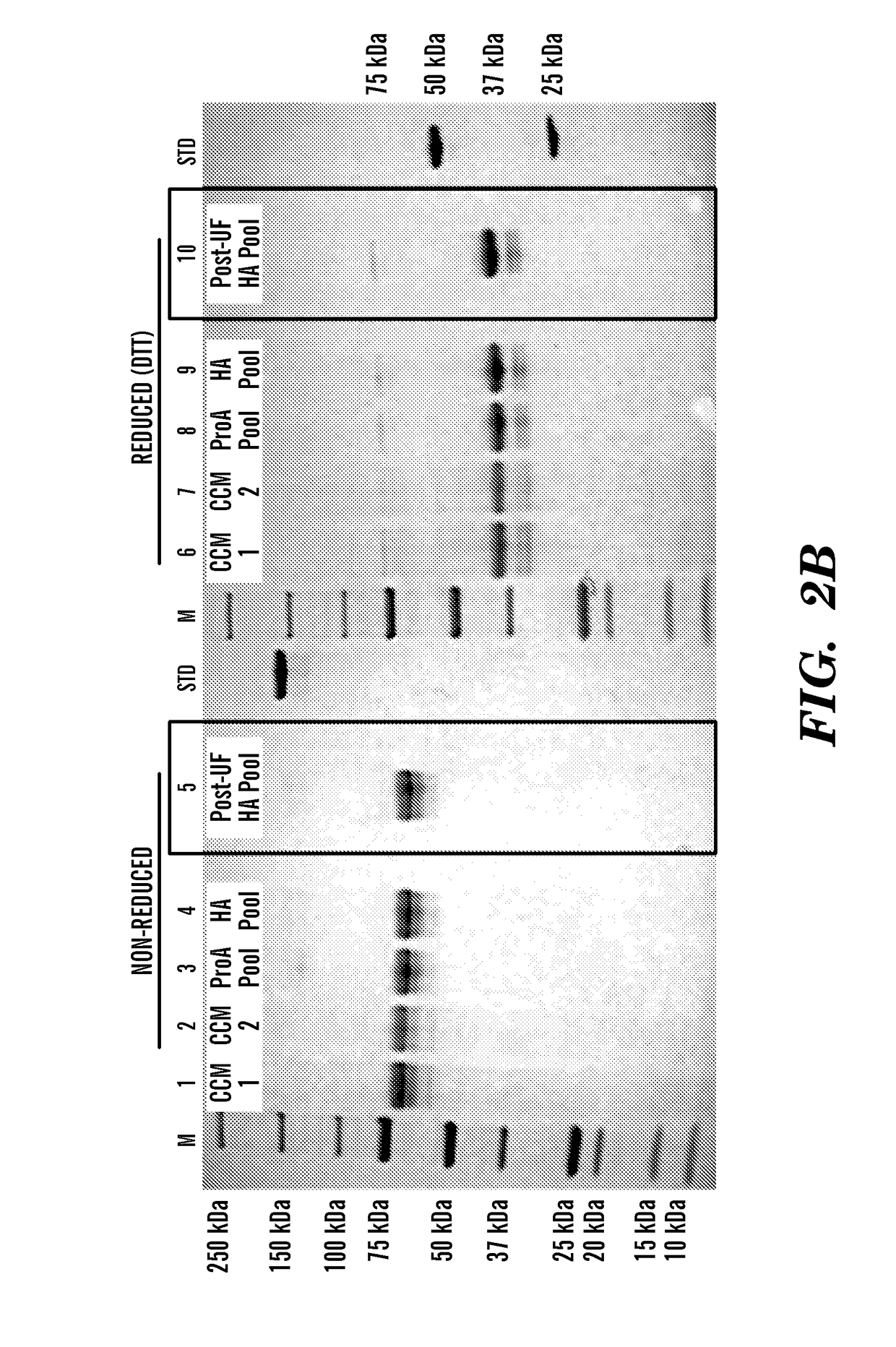
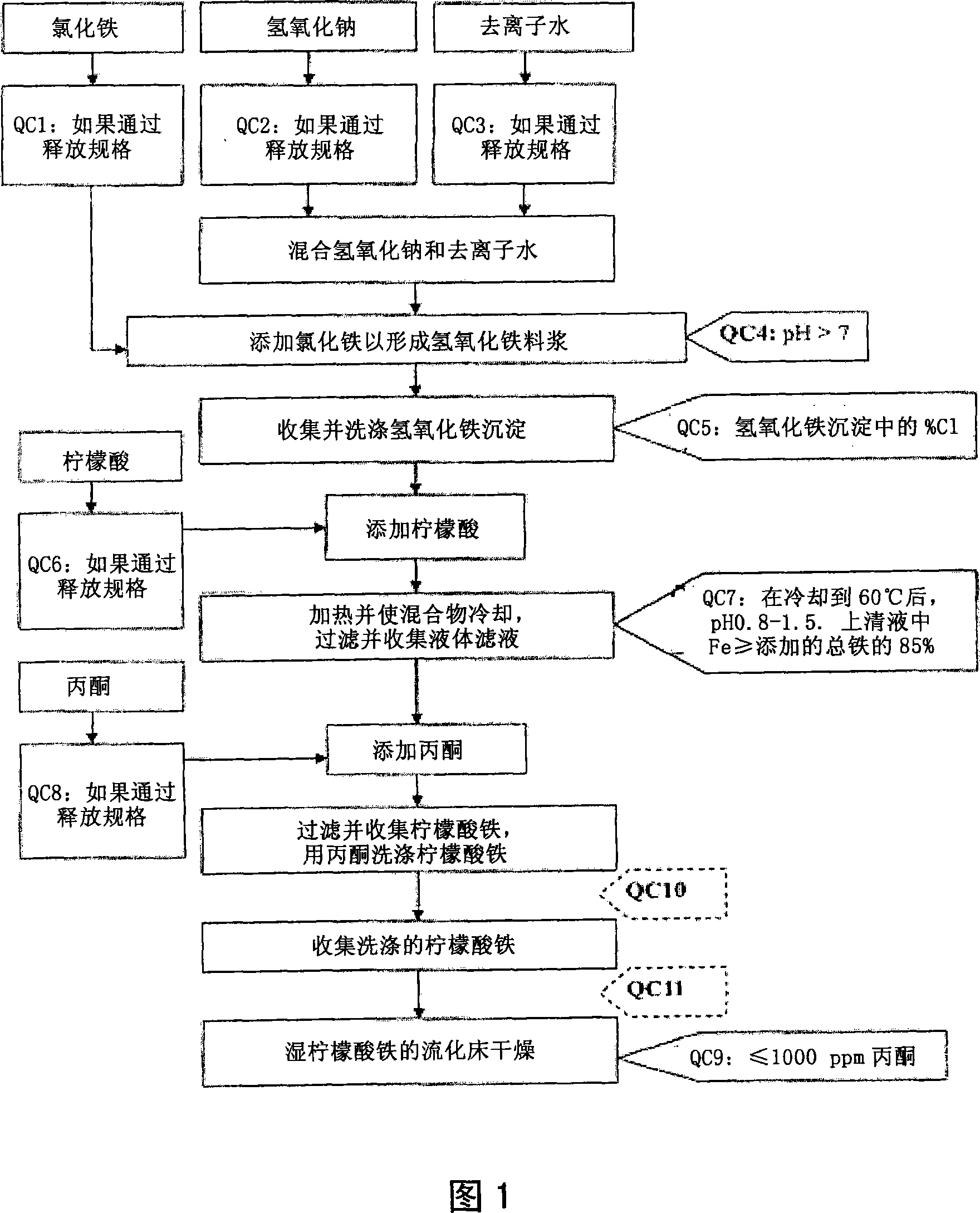
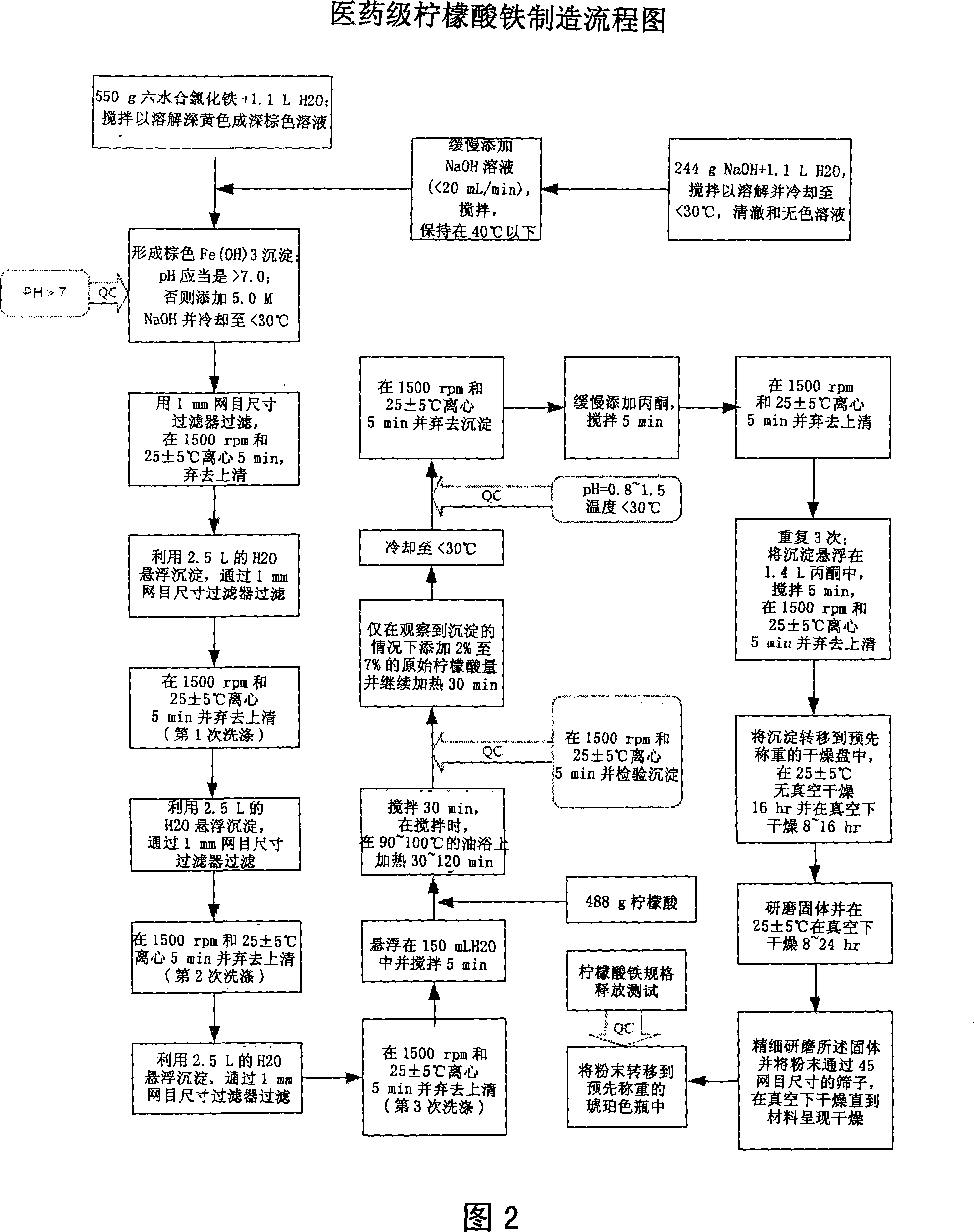
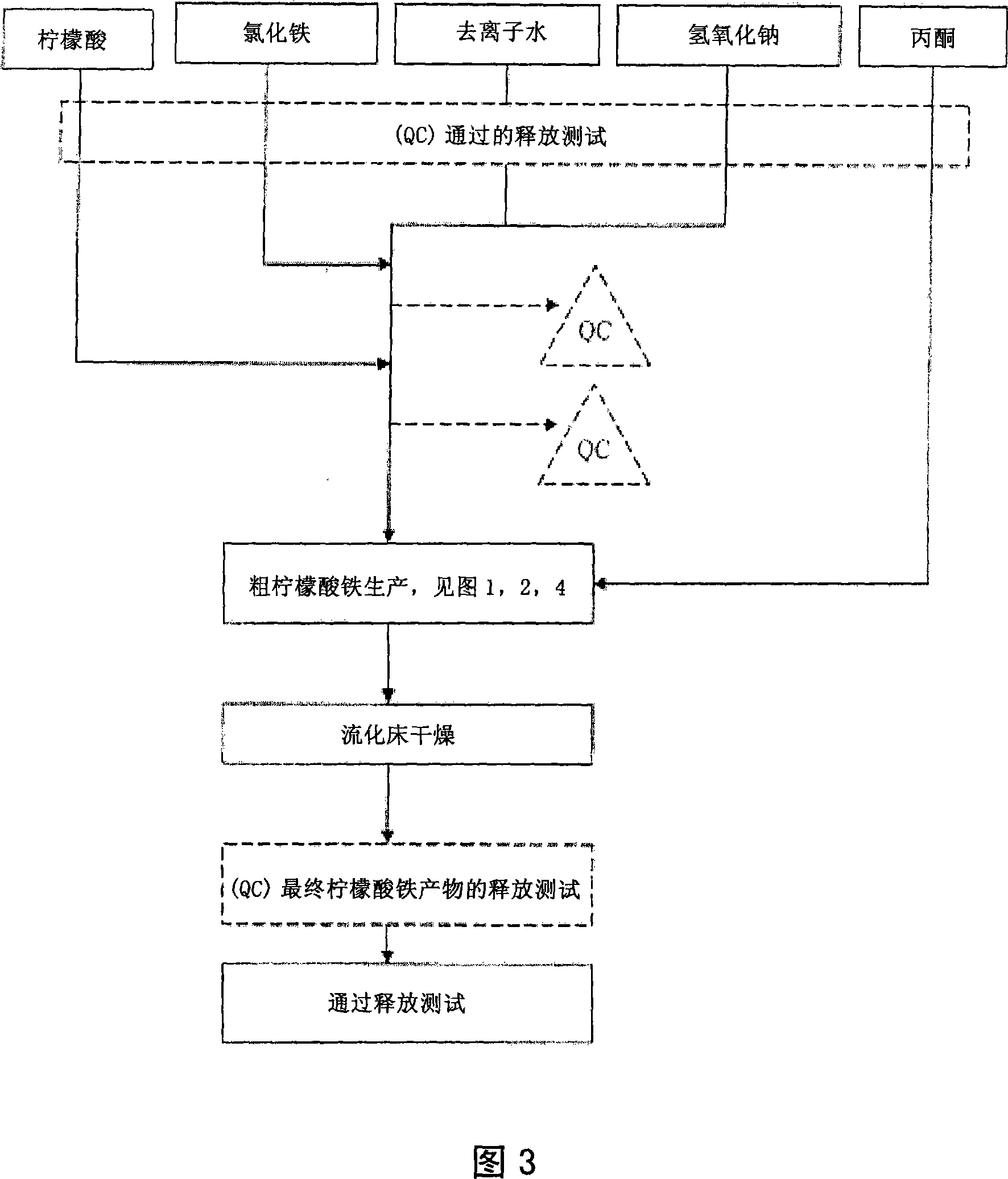
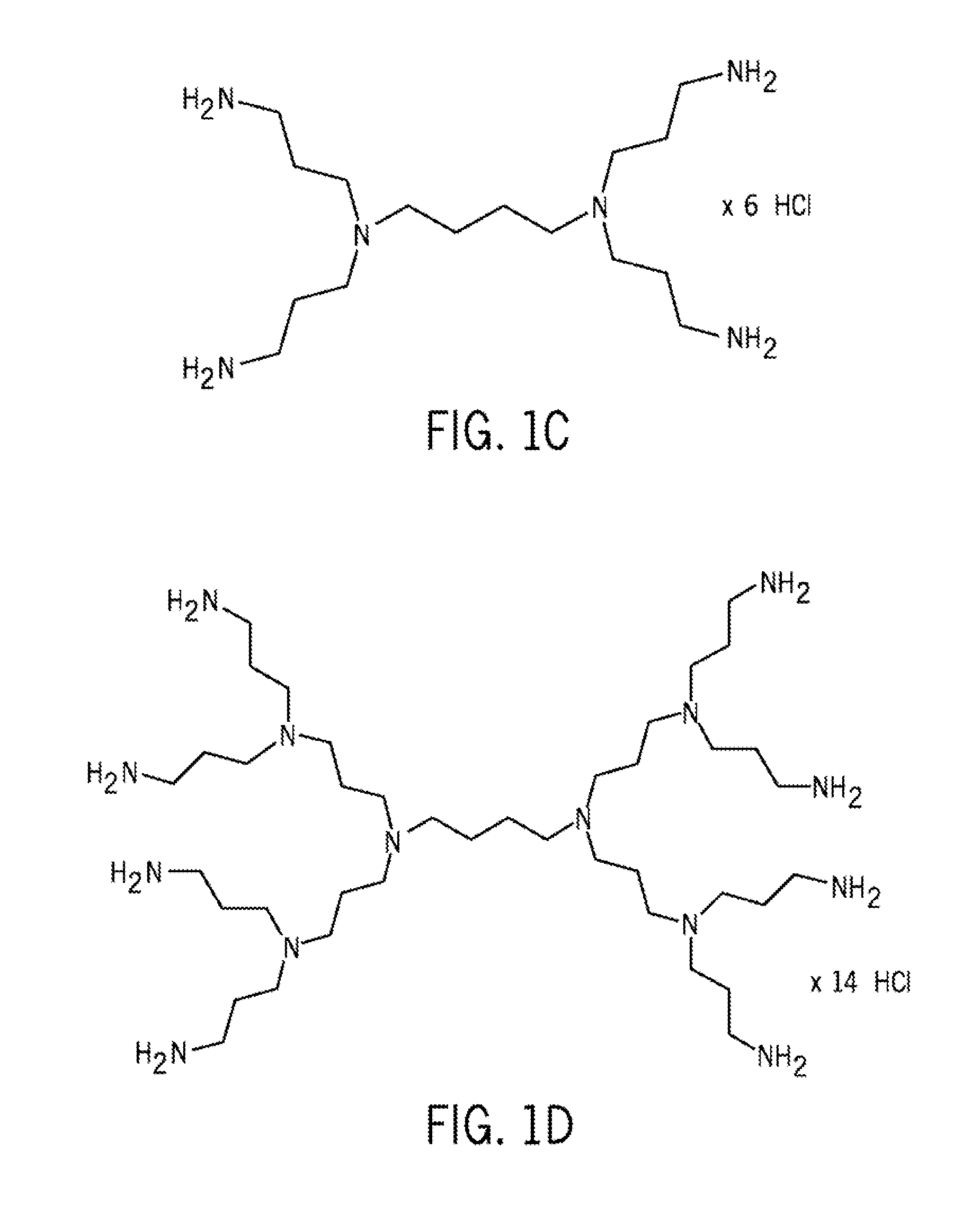
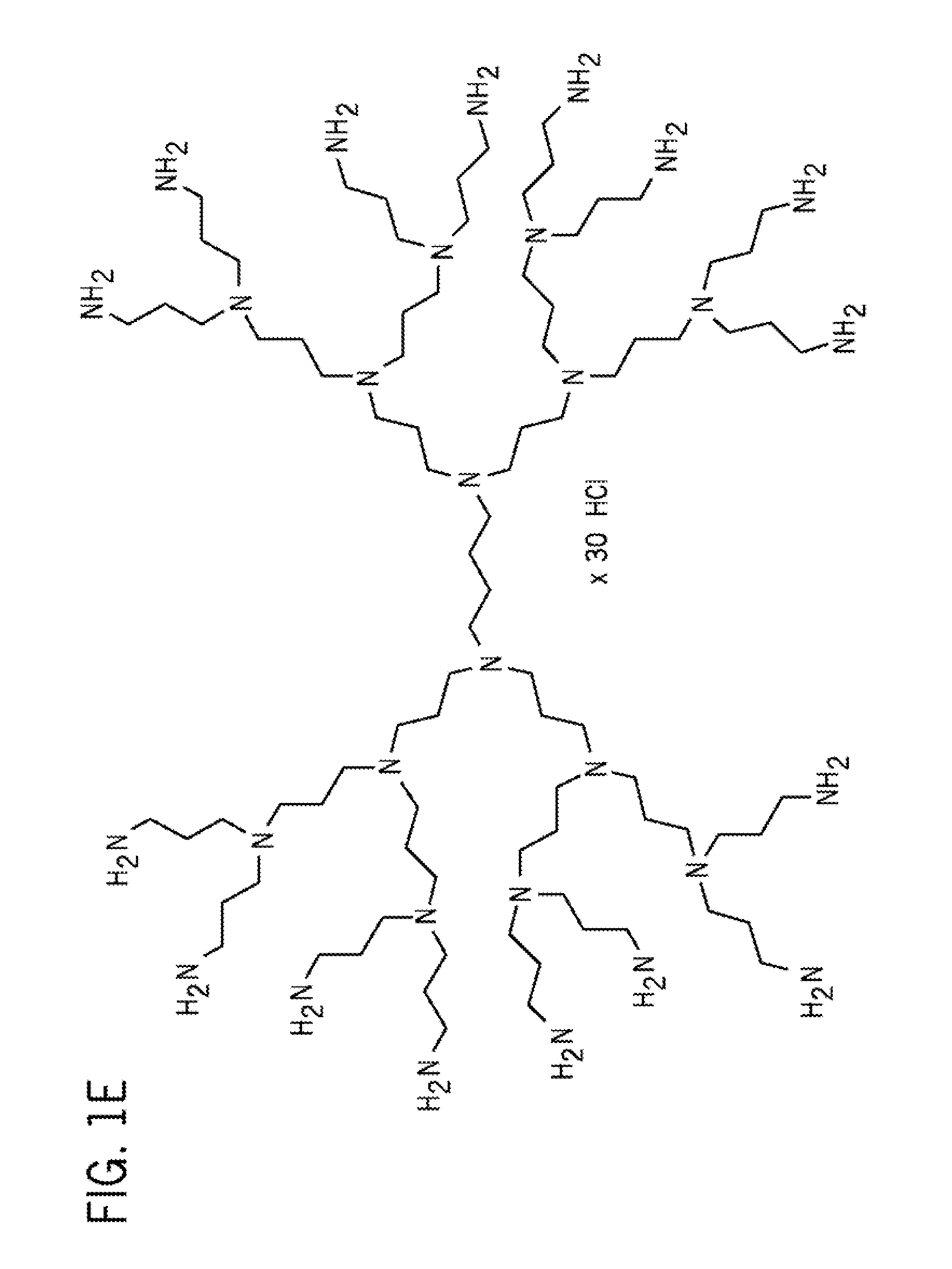
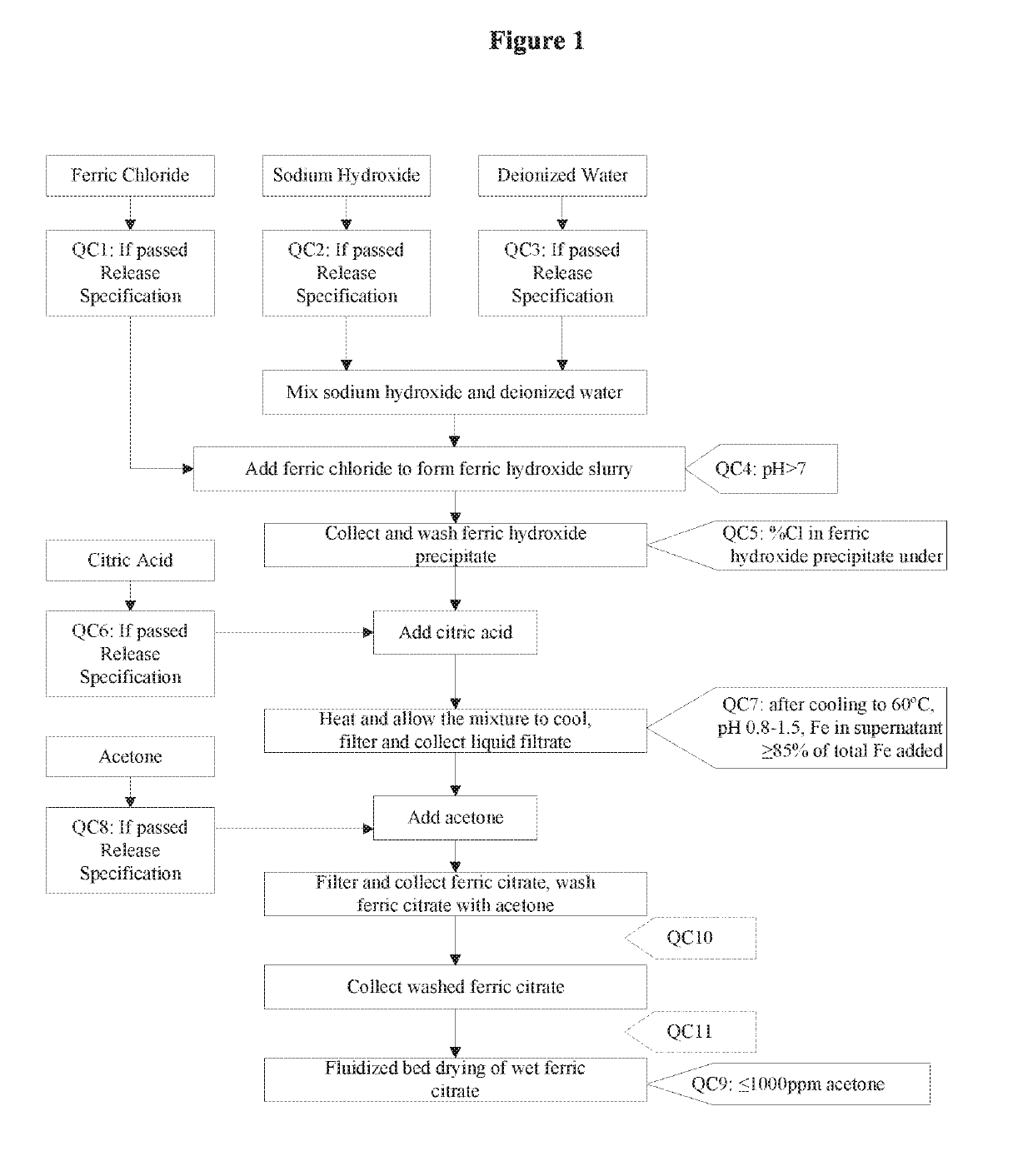
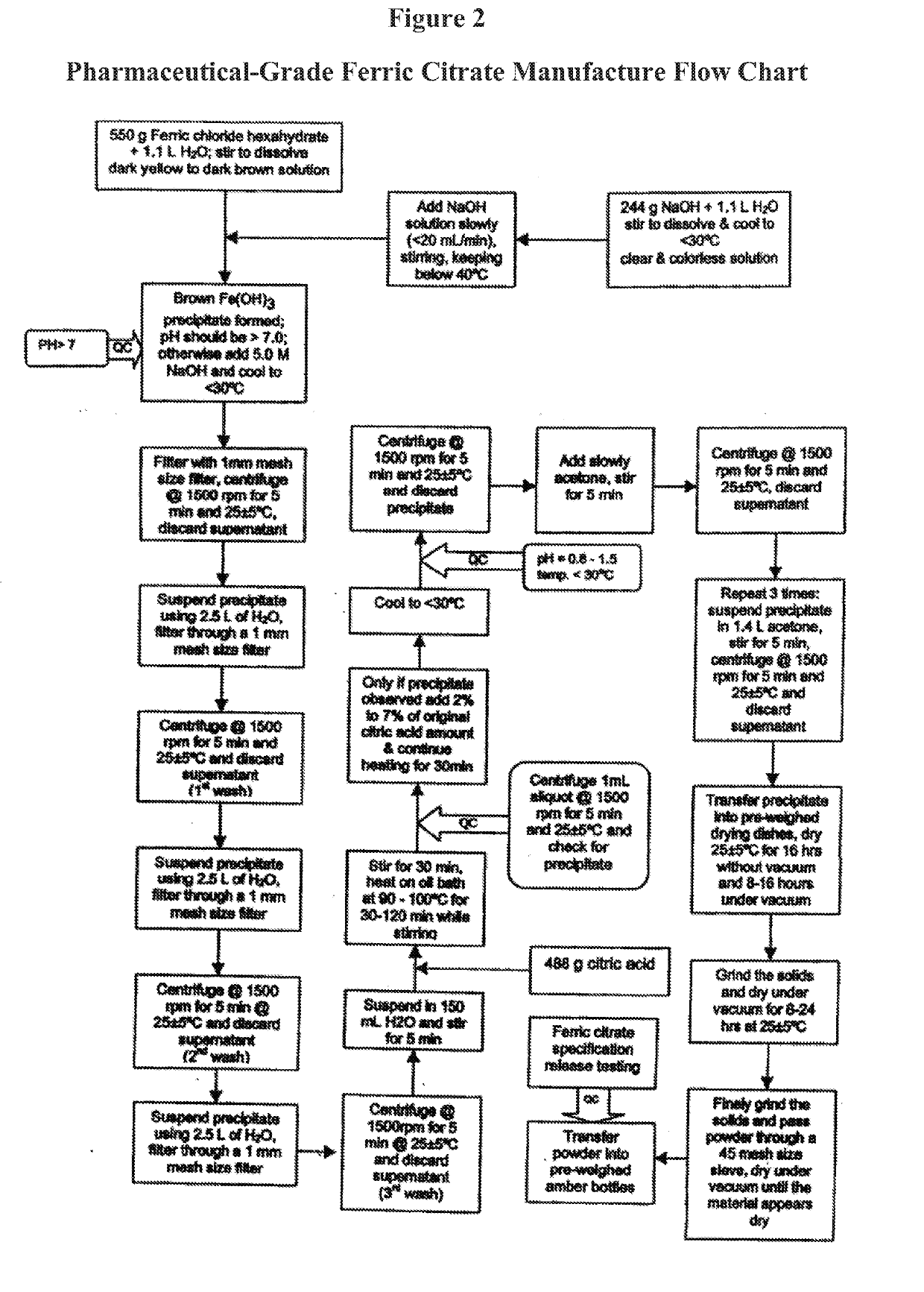
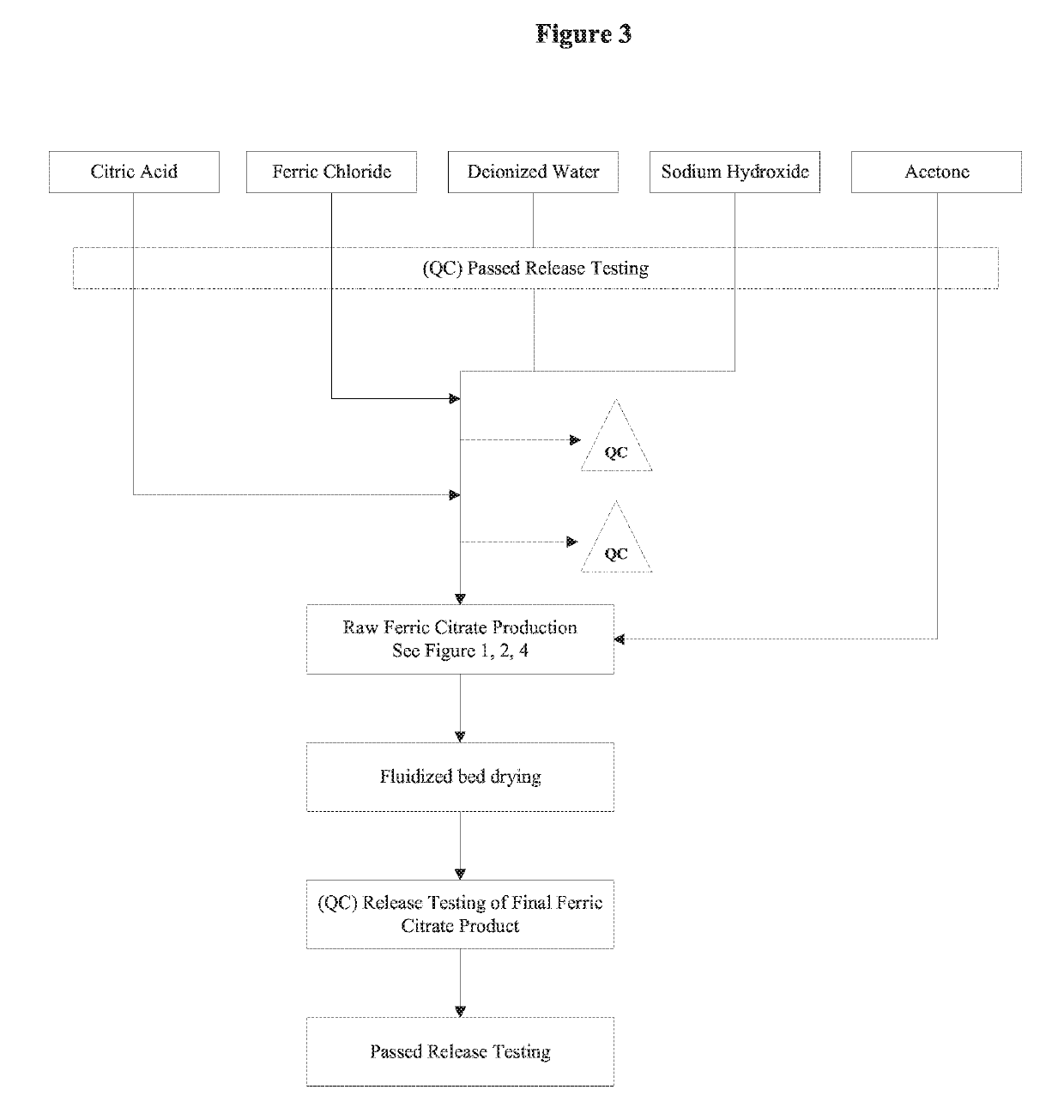
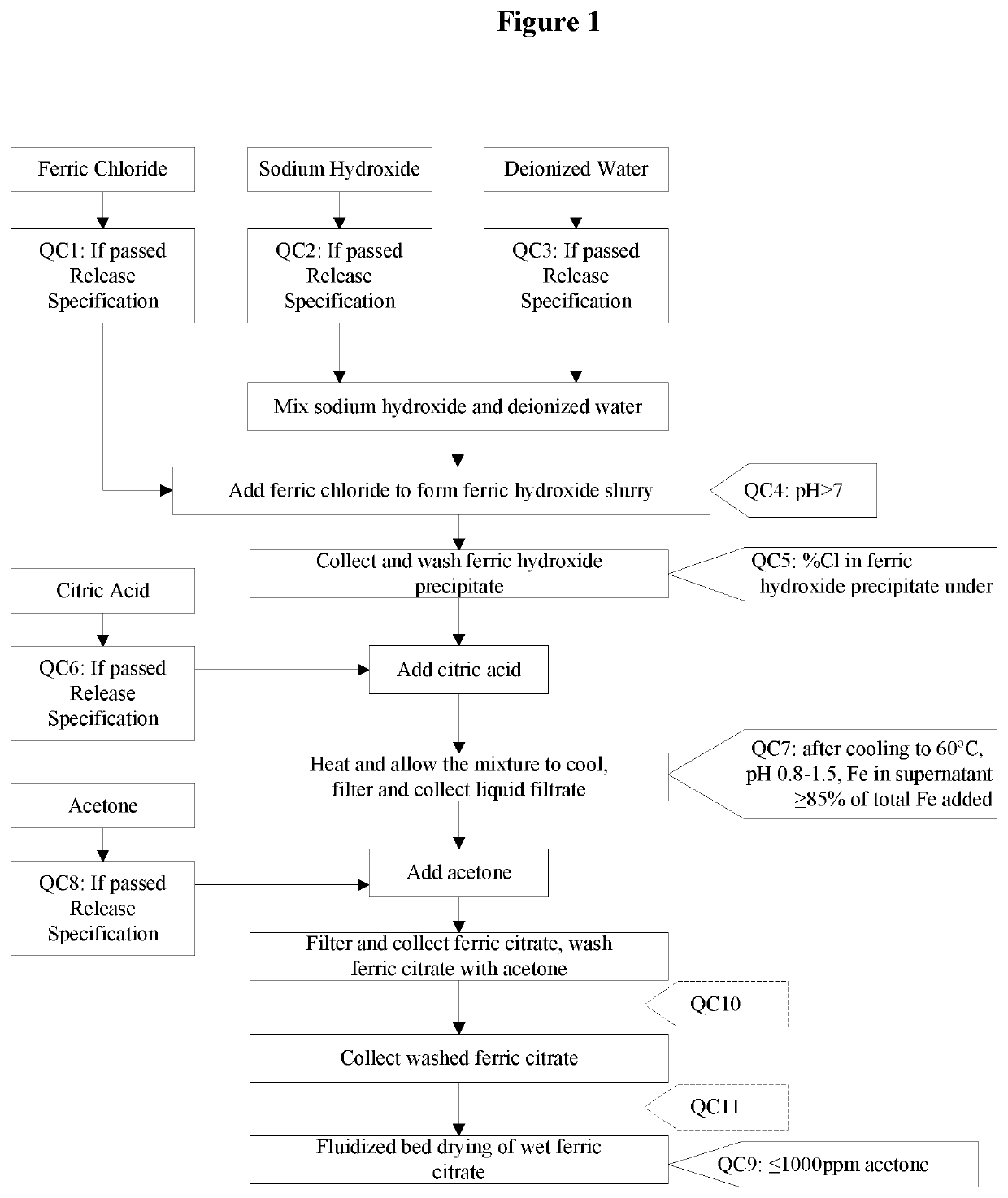
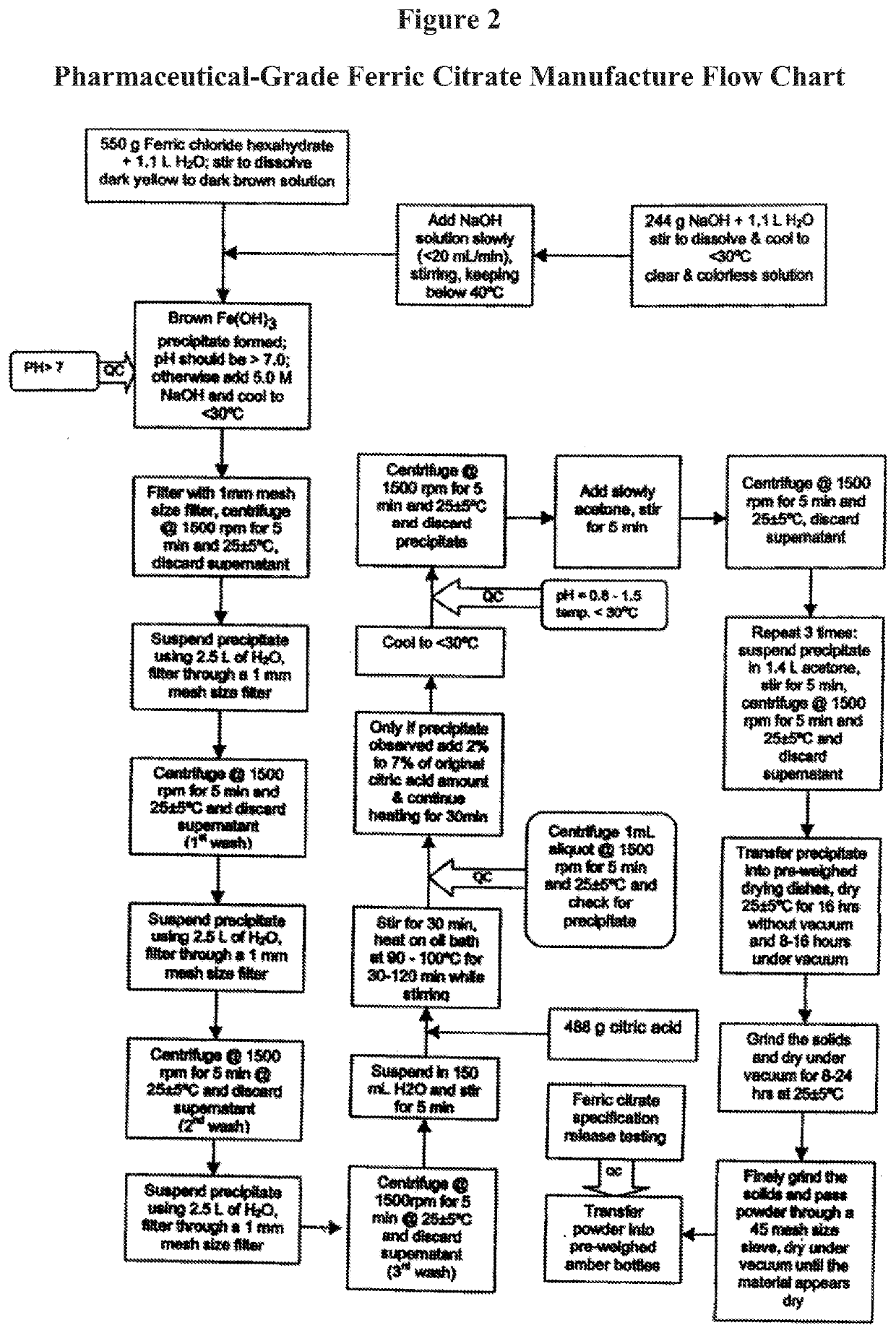
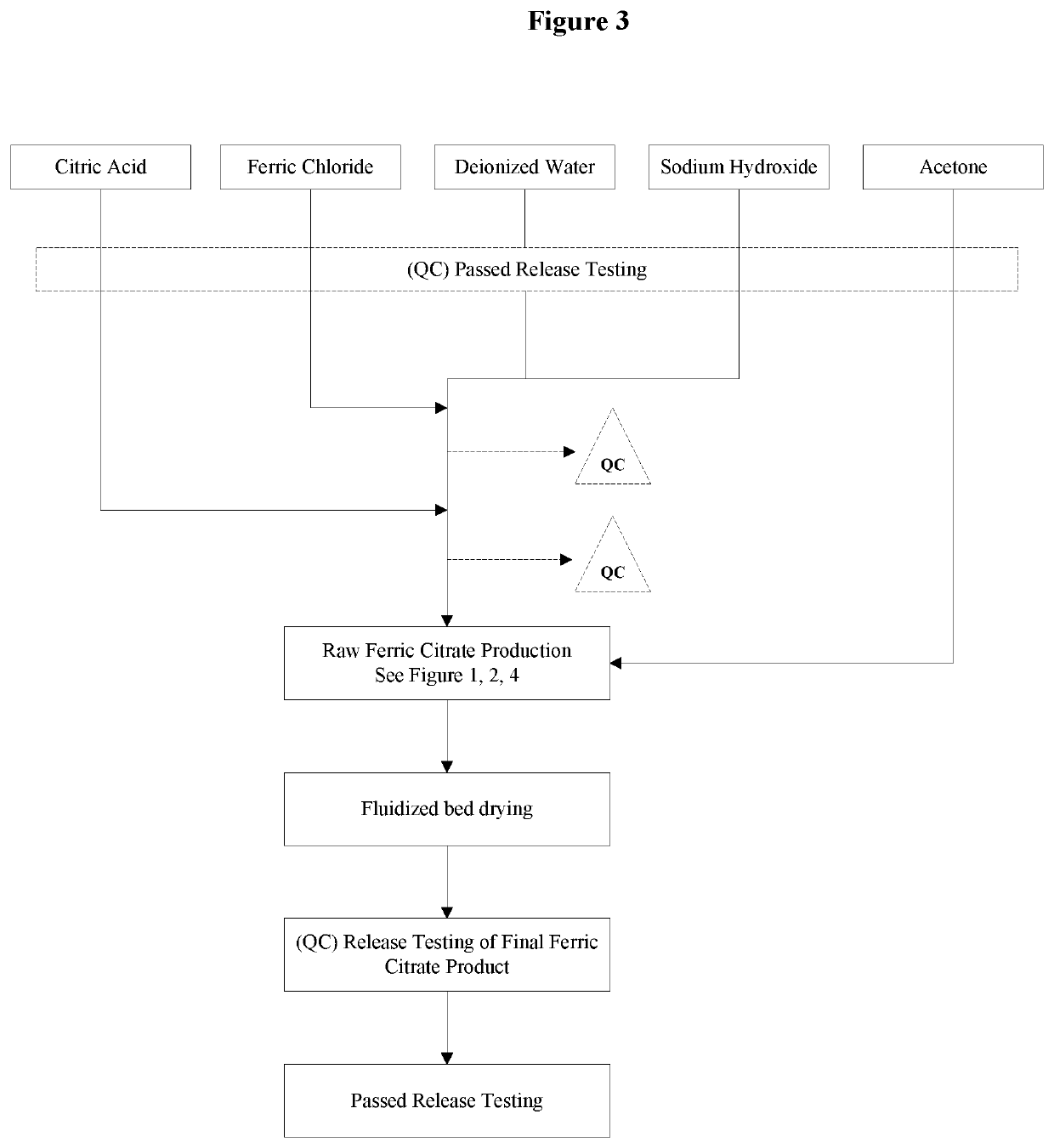
The present invention discloses a pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical-grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:PANION & BF BIOTECH INC
Fgf23 fusion proteins
ActiveUS20170226172A1Peptide/protein ingredientsAntibody mimetics/scaffoldsSerum phosphateHeterologous
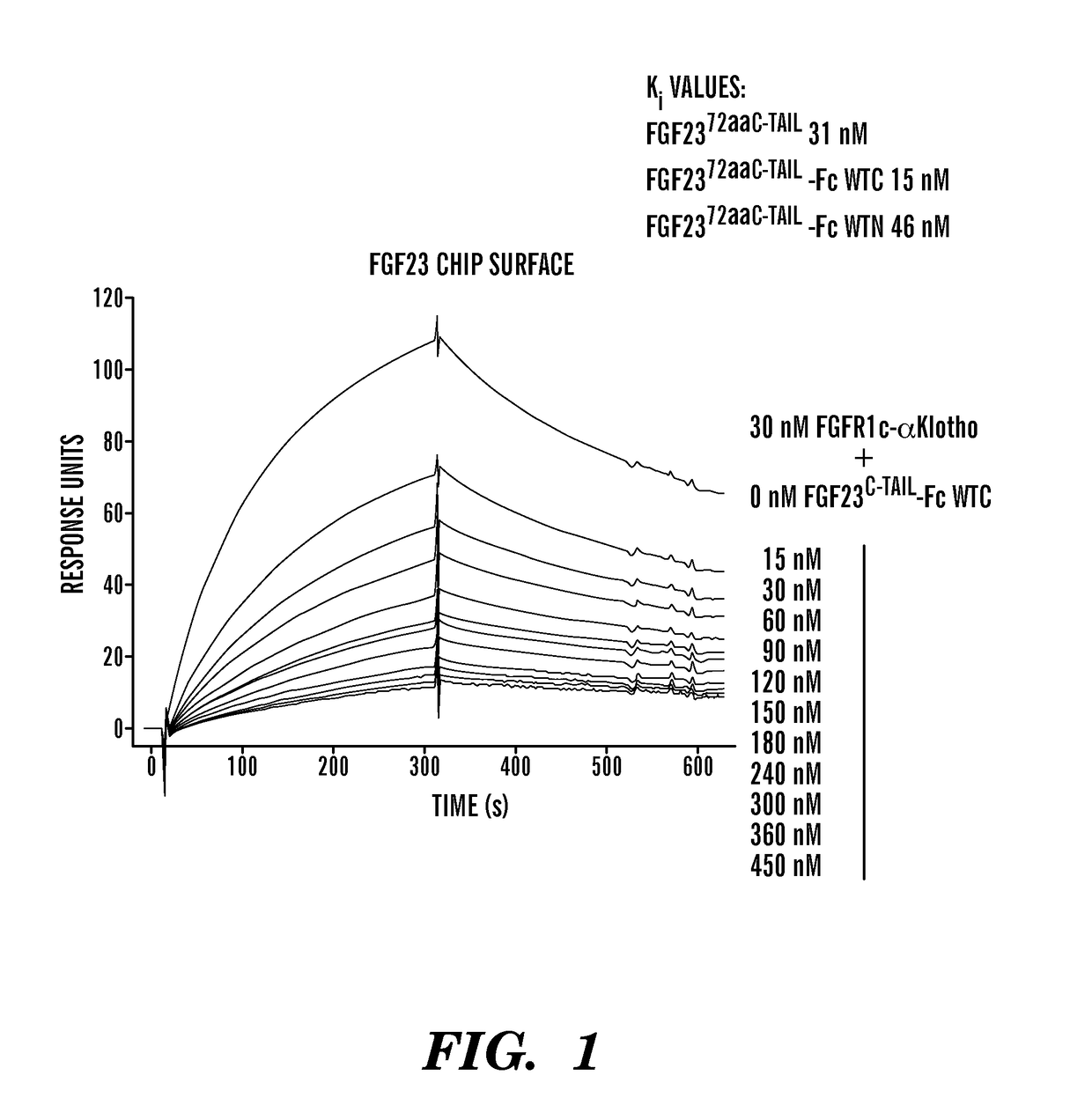
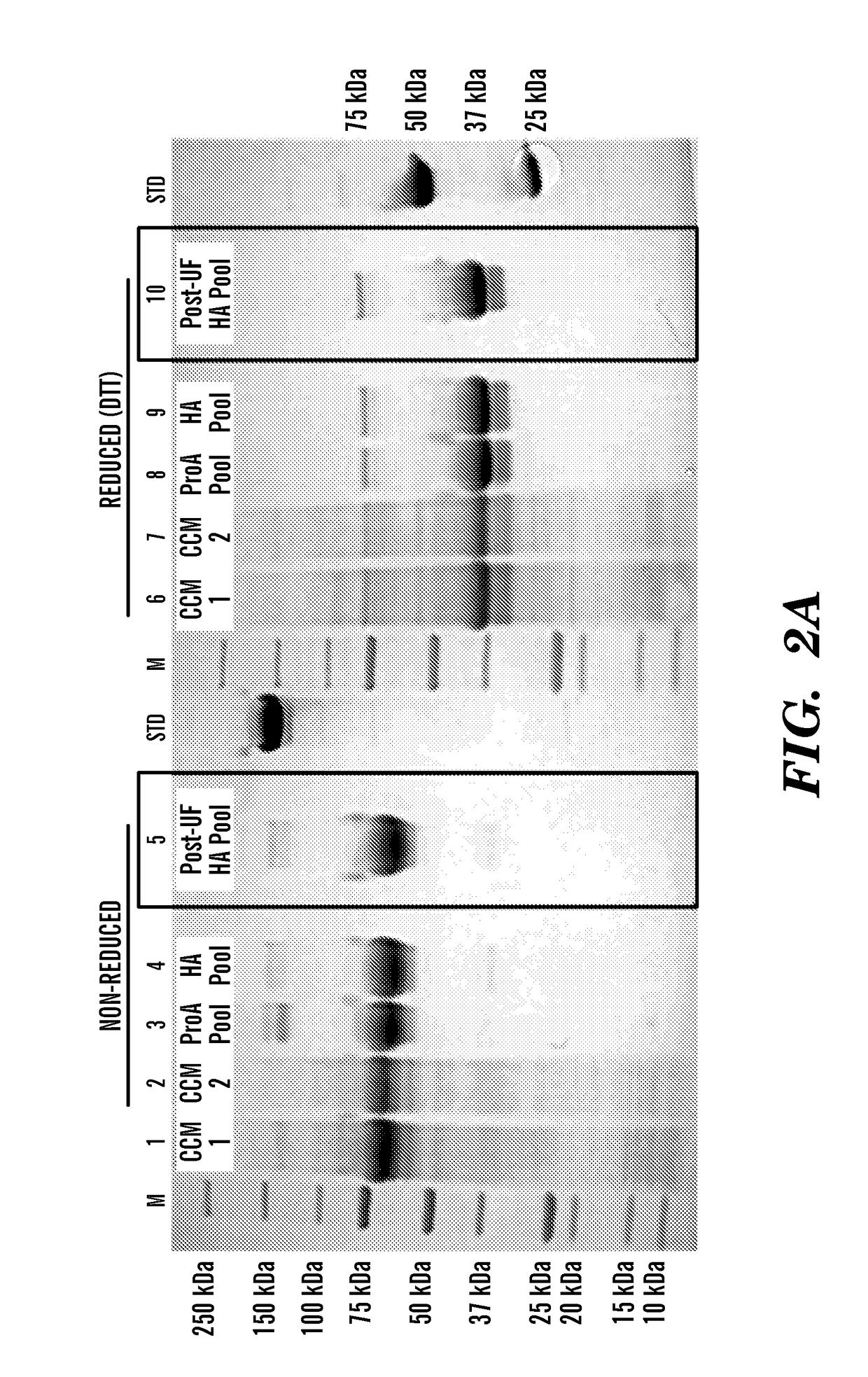
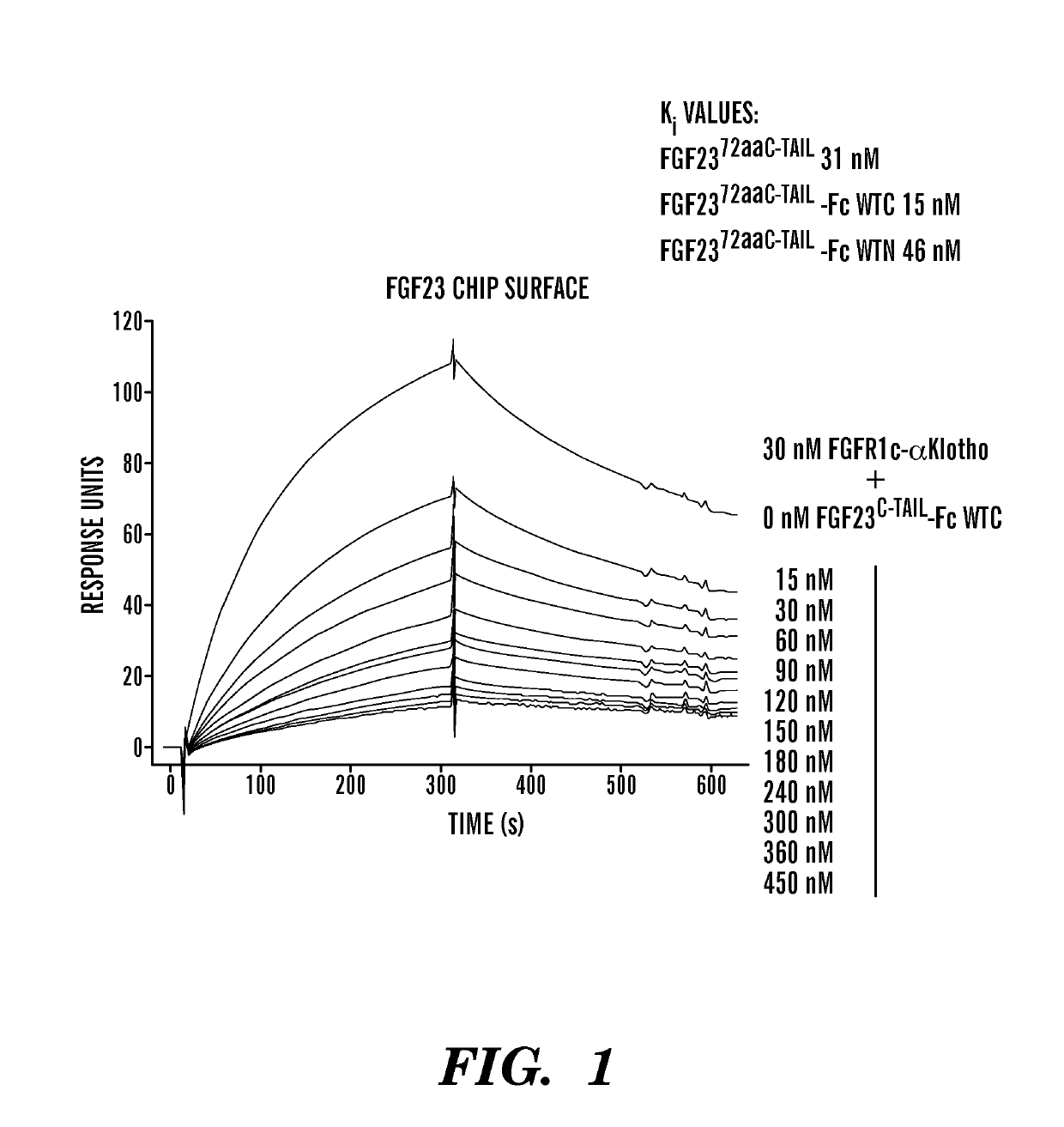
Disclosed herein are FGF23 c-tail fusion proteins, pharmaceutical compositions comprising the FGF23 c-tail fusion proteins, and methods of treatment using the FGF23 c-tail fusion proteins. This application discloses fusion proteins comprising a FGF-23 c-tail protein fused to a heterologous amino acid sequence, wherein said fusion protein modulates serum phosphate levels but does not substantially modulate serum 1, 25 VitD levels. In some embodiments, the FGF-23 c-tail protein is fused to the heterologous amino acid sequence via a linker. This invention also encompasses vectors comprising the nucleic acids disclosed herein, and a host cell comprising the vector or polynucleotides encoding the proteins of the invention.
Owner:PFIZER INC +1
Ferric organic compounds, uses thereof and methods of making same
The present invention discloses a pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical -grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:GLOBOASIA
Serum phosphate adsorbing agent and preparation method thereof and adsorption column for blood perfusion
ActiveCN104258829ARemissionRelieve painOther chemical processesOther blood circulation devicesCelluloseSerum phosphate
The invention provides a serum phosphate adsorbing agent; a hydrophilic carrier is immobilized by ligands capable of capturing phosphate ions to obtain the serum phosphate adsorbing agent; the hydrophilic carrier is selected from one or more out of cellulose, agarose and polyvinyl alcohol microsphere, and the ligands capable of capturing phosphate ions refer to amino polymers. The invention also provides a preparation method of the serum phosphate adsorbing agent and an adsorption column for blood perfusion, prepared by the serum phosphate adsorbing agent. Compared with medicines which are orally taken to act on the human body, the serum phosphate adsorbing agent can directly remove the phosphate ions in blood through blood circulation, so as to immediately alleviate the illness state of a patient; and the serum phosphate adsorbing agent can be combined with hemodialysis, oral taking pain of the patient each day can be reduced, and the result caused by a situation that the patient forgets to take the medicine can be avoided.
Owner:JAFRON BIOMEDICAL
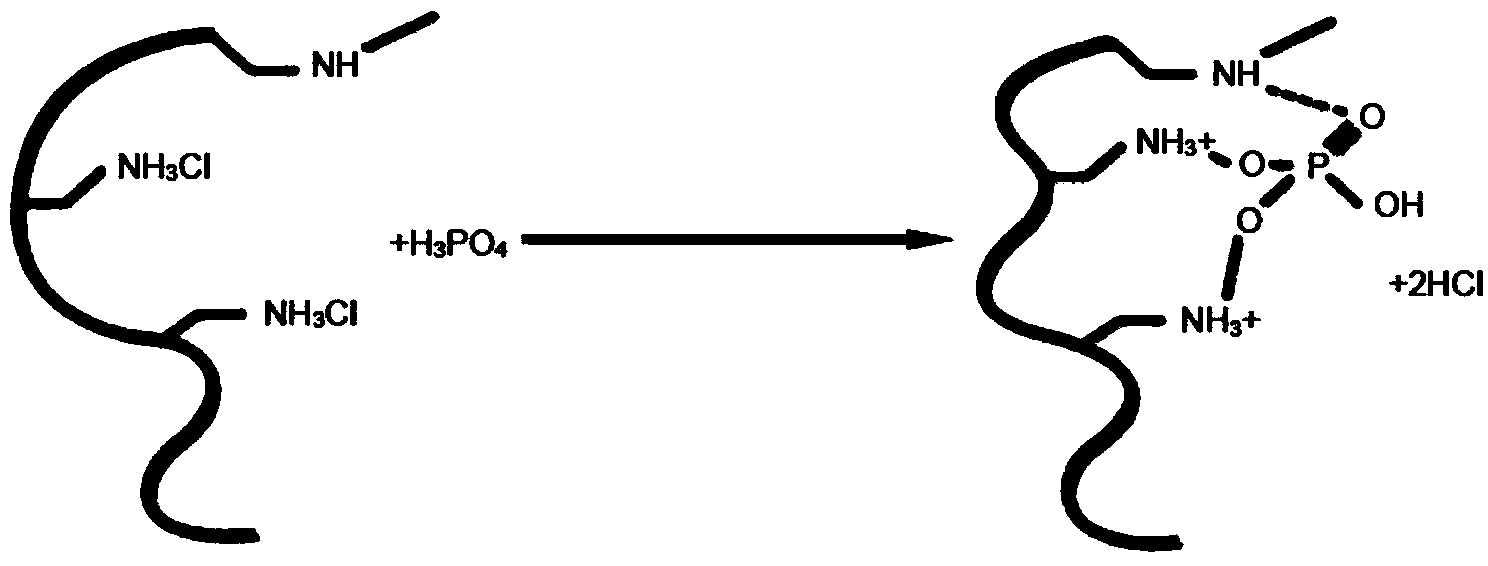
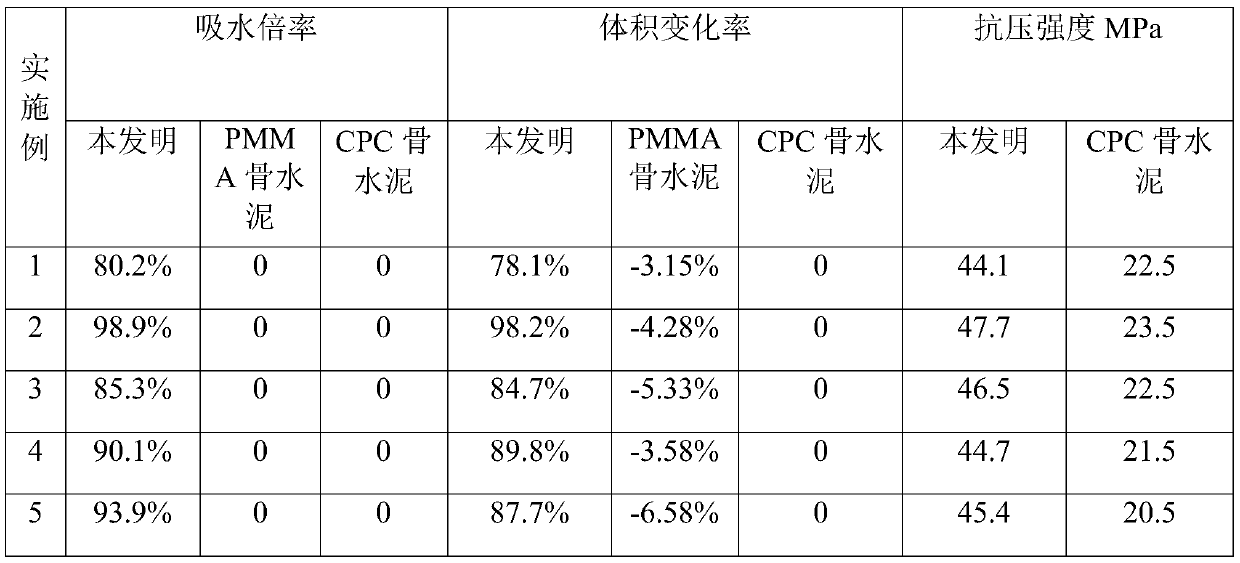
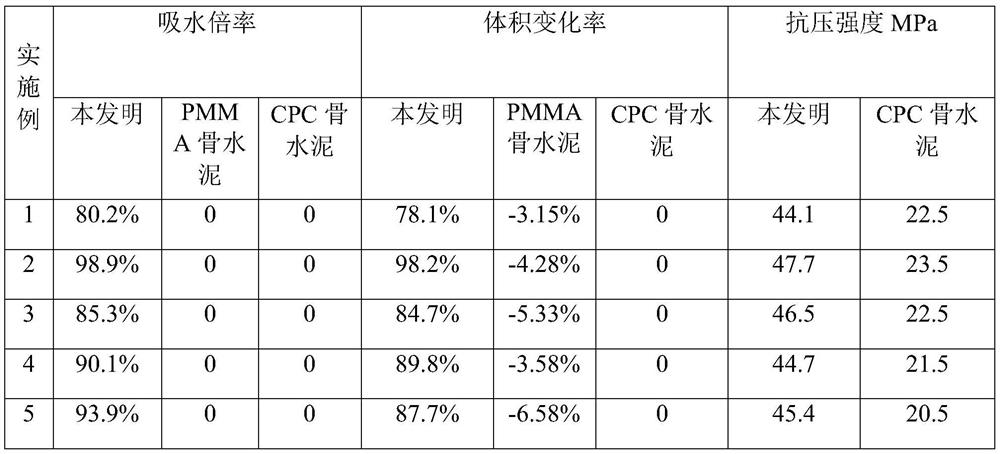
Self-expanding composite bone cement and preparation method thereof
ActiveCN111467564AImprove mechanical propertiesEnhanced interface bindingPharmaceutical delivery mechanismTissue regenerationSerum phosphateMicrosphere
The invention discloses self-expanding composite bone cement and a preparation method thereof. The self-expanding composite bone cement is prepared by mixing a solid-phase component and a liquid-phasecomponent according to a mass-volume ratio of (1g: 1.5 ml) to (1g: 7.2 ml); wherein the solid phase is prepared from the following raw materials in percentage by mass: 30%-70% of polymethyl methacrylate-acrylic acid-anhydrous calcium hydrophosphate copolymer microspheres, 15%-35% of anhydrous calcium hydrophosphate and 15%-35% of tetracalcium phosphate, and the sum of the mass percentages of thecomponents is 100%; and the bone cement liquid phase is any one of distilled water, blood, normal saline, dilute acid, serum and phosphate solution. The prepared self-expanding composite bone cement has high water absorption expansion rate and good mechanical properties, and is expected to become promising bone cement for clinical application.
Owner:XIAN UNIV OF TECH
Methods of reducing phosphate absorption
ActiveUS20100166760A1Eliminate side effectsReduce hyperphosphatemiaMetabolism disorderImmunoglobulins against cell receptors/antigens/surface-determinantsSerum phosphateHuman animal
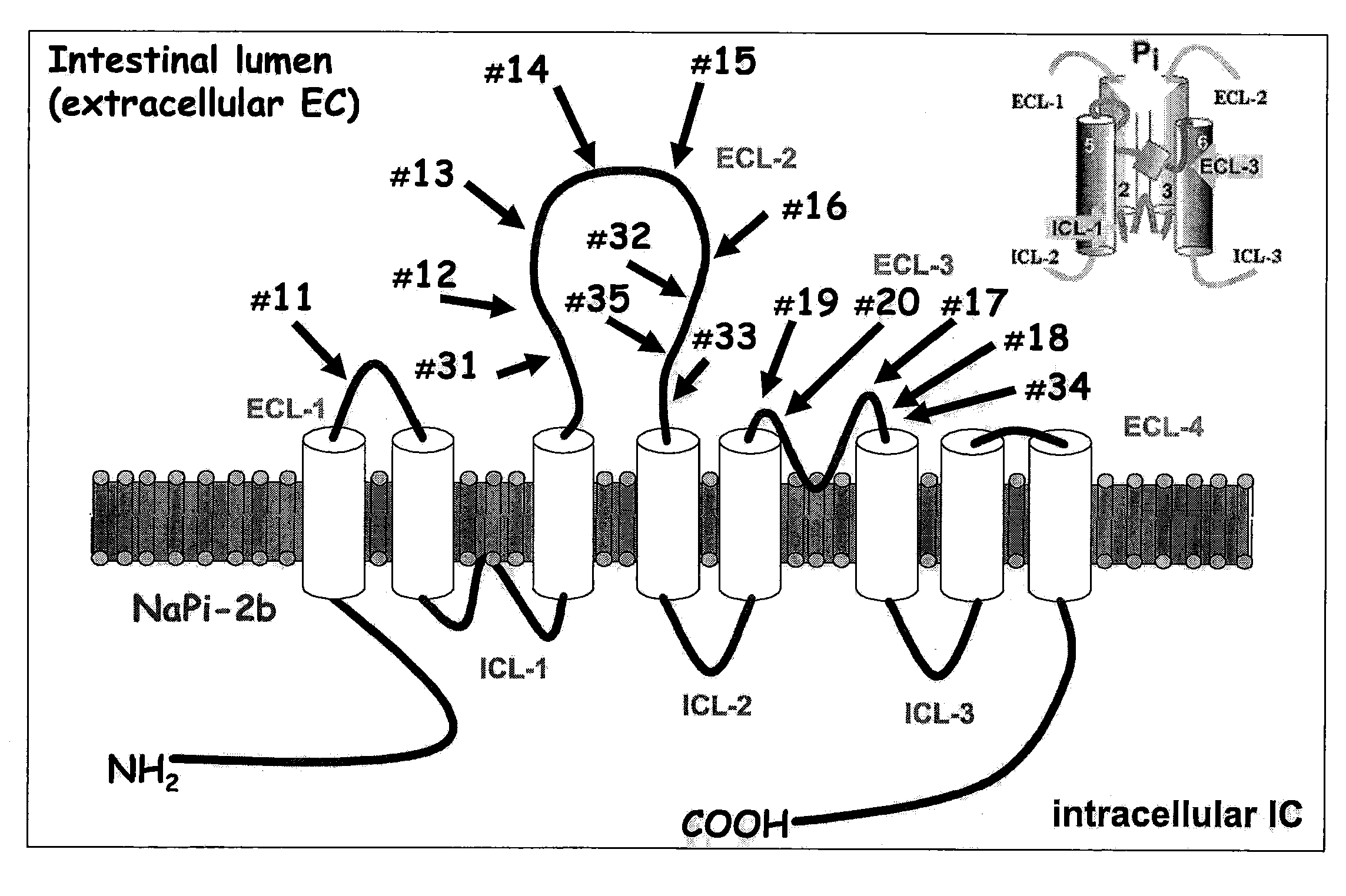
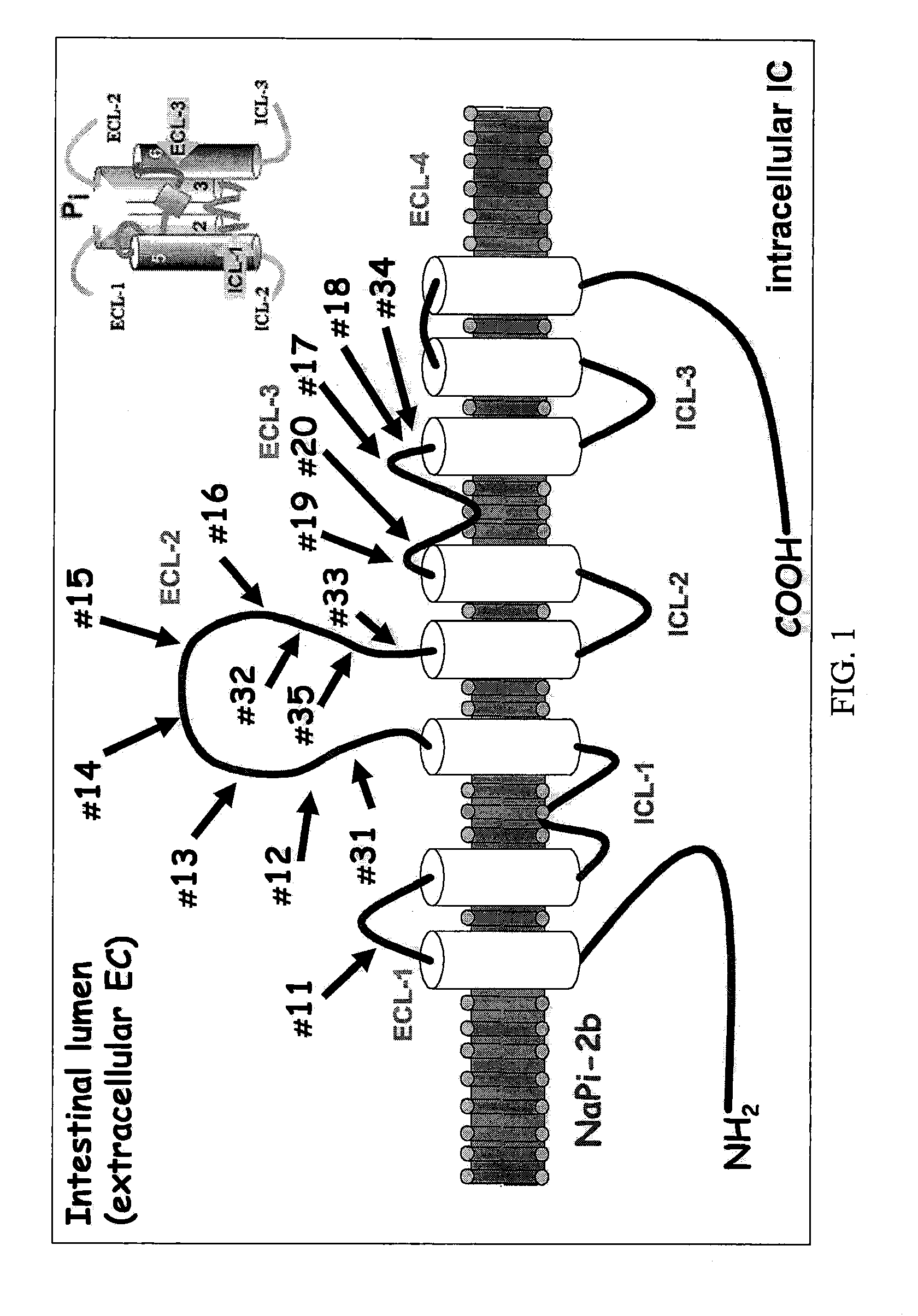
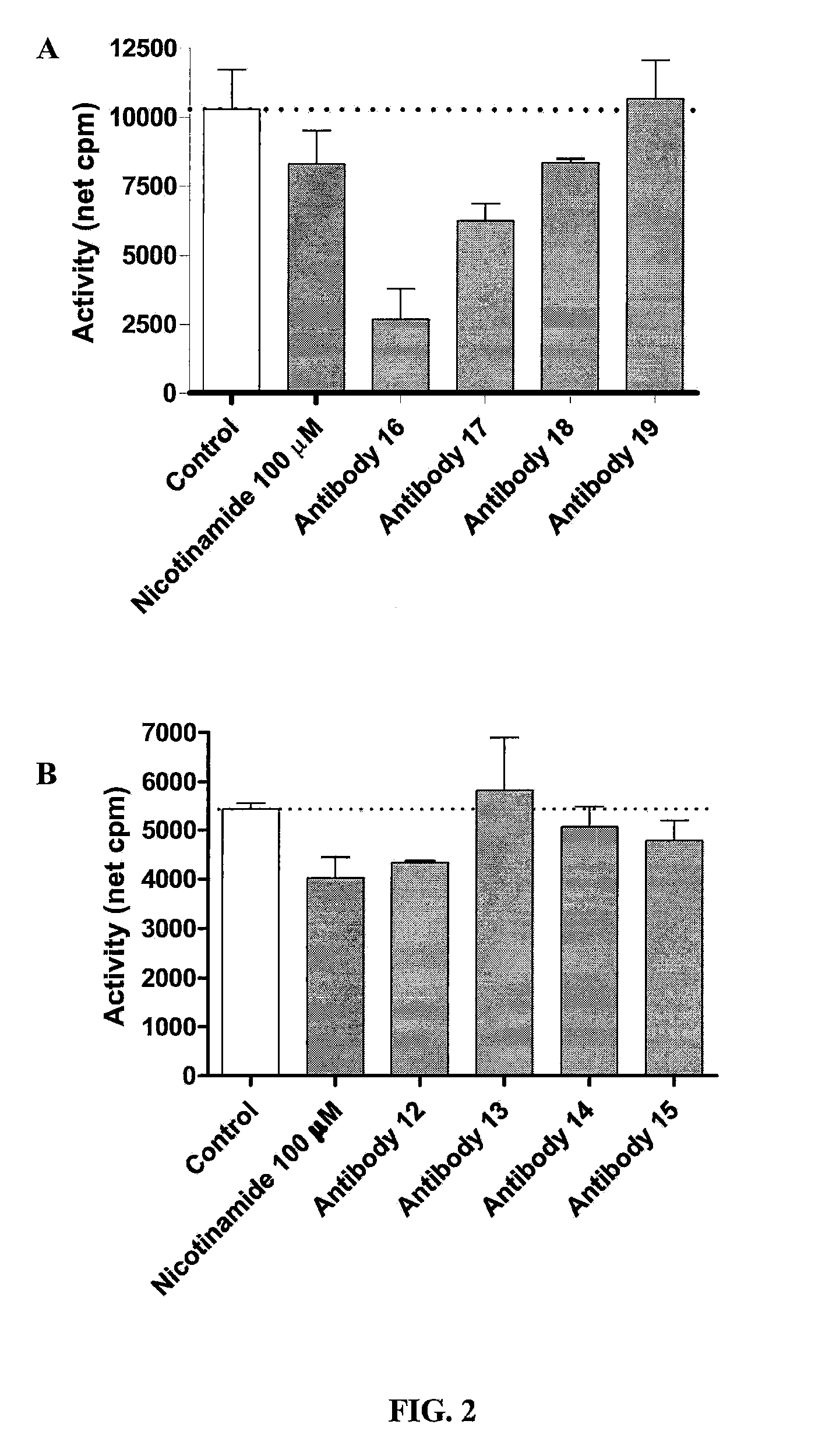
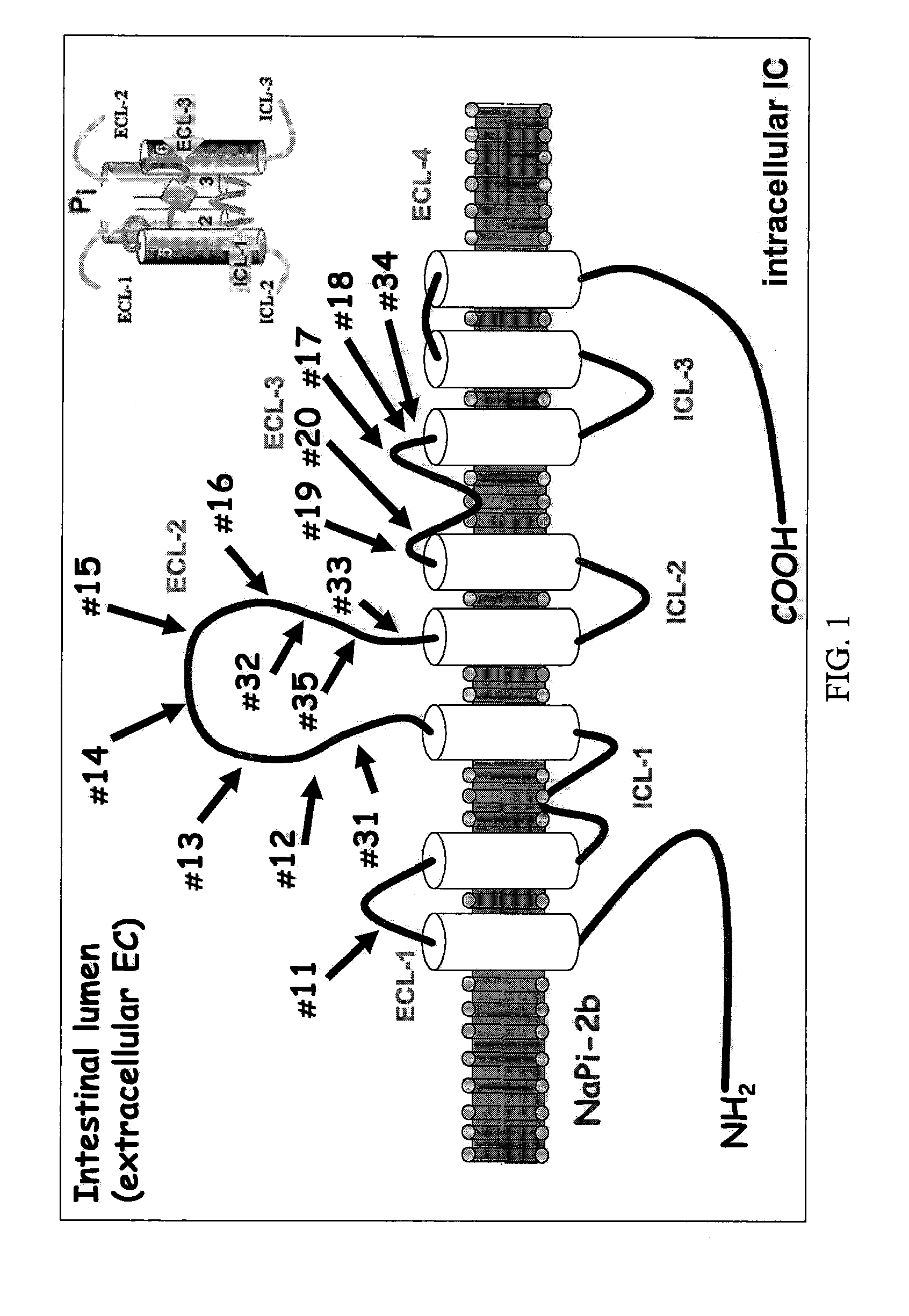
A method for reducing phosphate absorption in a human or non-human animal subject at risk of developing or having developed hyperphosphatemia is disclosed. The method includes the step of administering orally to the subject an anti-intestinal sodium phosphate cotransporter type 2B (Npt2B) antibody in an amount effective to reduce or maintain the serum phosphate concentration in the subject.
Owner:CYCTOCHROMA INC +1
Phosphate binder and preparation method thereof
The invention provides a phosphate binder and a preparation method thereof and in particular provides a method for preparing the phosphate binder. The method comprises the following steps: (i) providing a mixed solution, wherein the mixed solution comprises (a) a carbohydrate; (b) citric acid, citrate, carbonate or a combination thereof, and (c) water; and (ii) adding an iron salt and an insoluble solvent into the mixed solution under alkaline conditions, producing a precipitate, and separating the precipitate, thereby obtaining the phosphate binder, wherein the pH value of the alkaline condition is 4-13. According to the method disclosed by the invention, the phosphate binder is capable of obviously reducing the serum phosphate concentration.
Owner:CINKATE PHARMA INTERMEDIATES
Infusion fluid for dialysis patients
InactiveUS20070281985A1Prevents and alleviates anemiaImprove malnutritionBiocidePeptide/protein ingredientsCysteine thiolateTryptophan
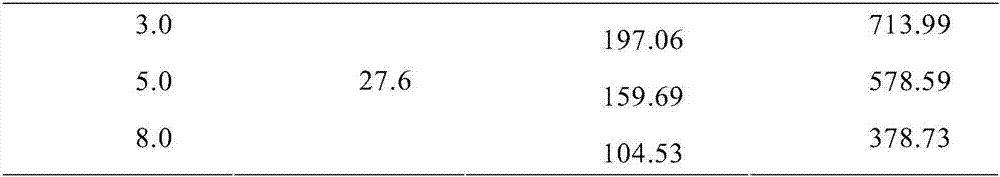
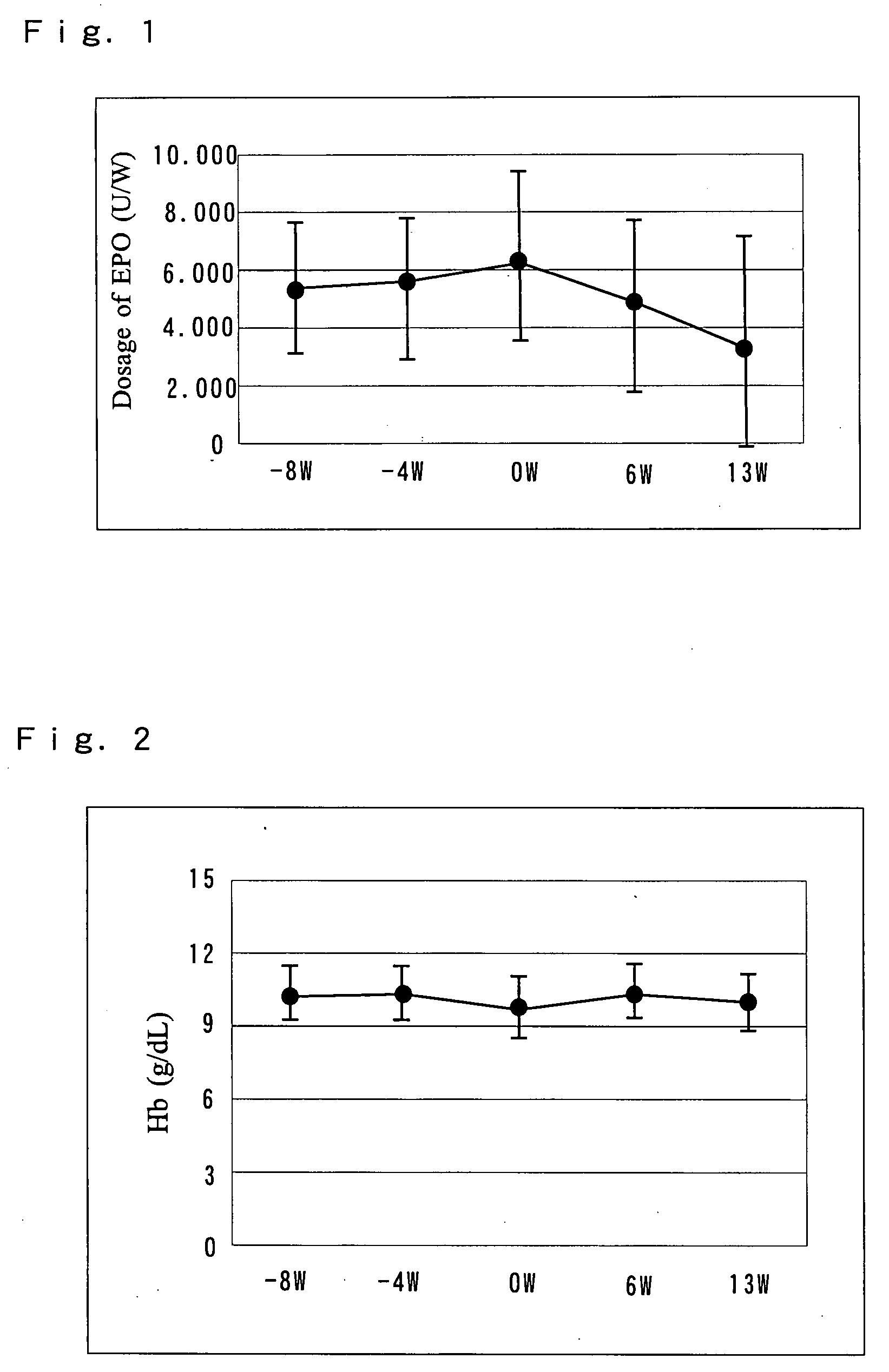
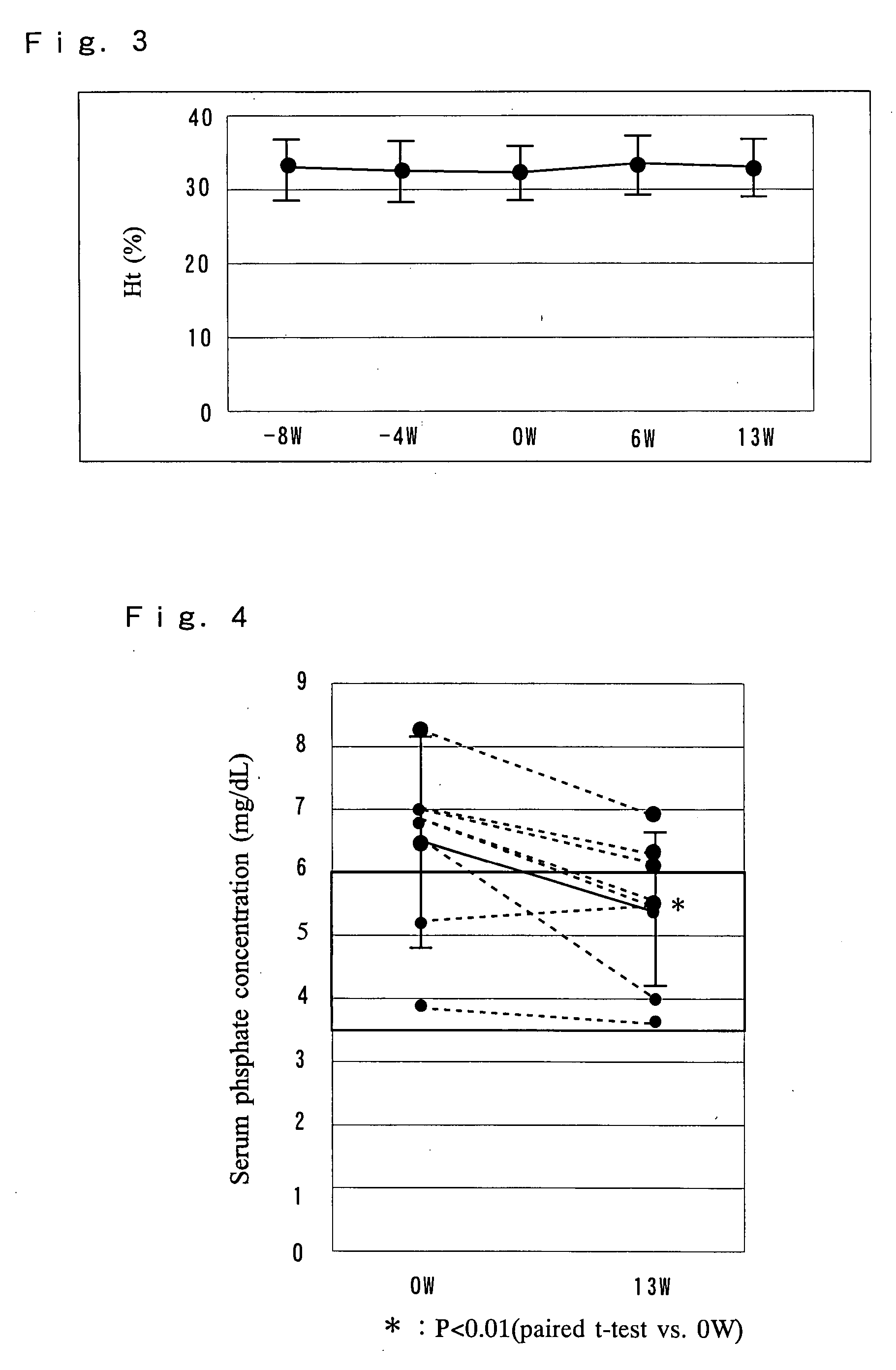
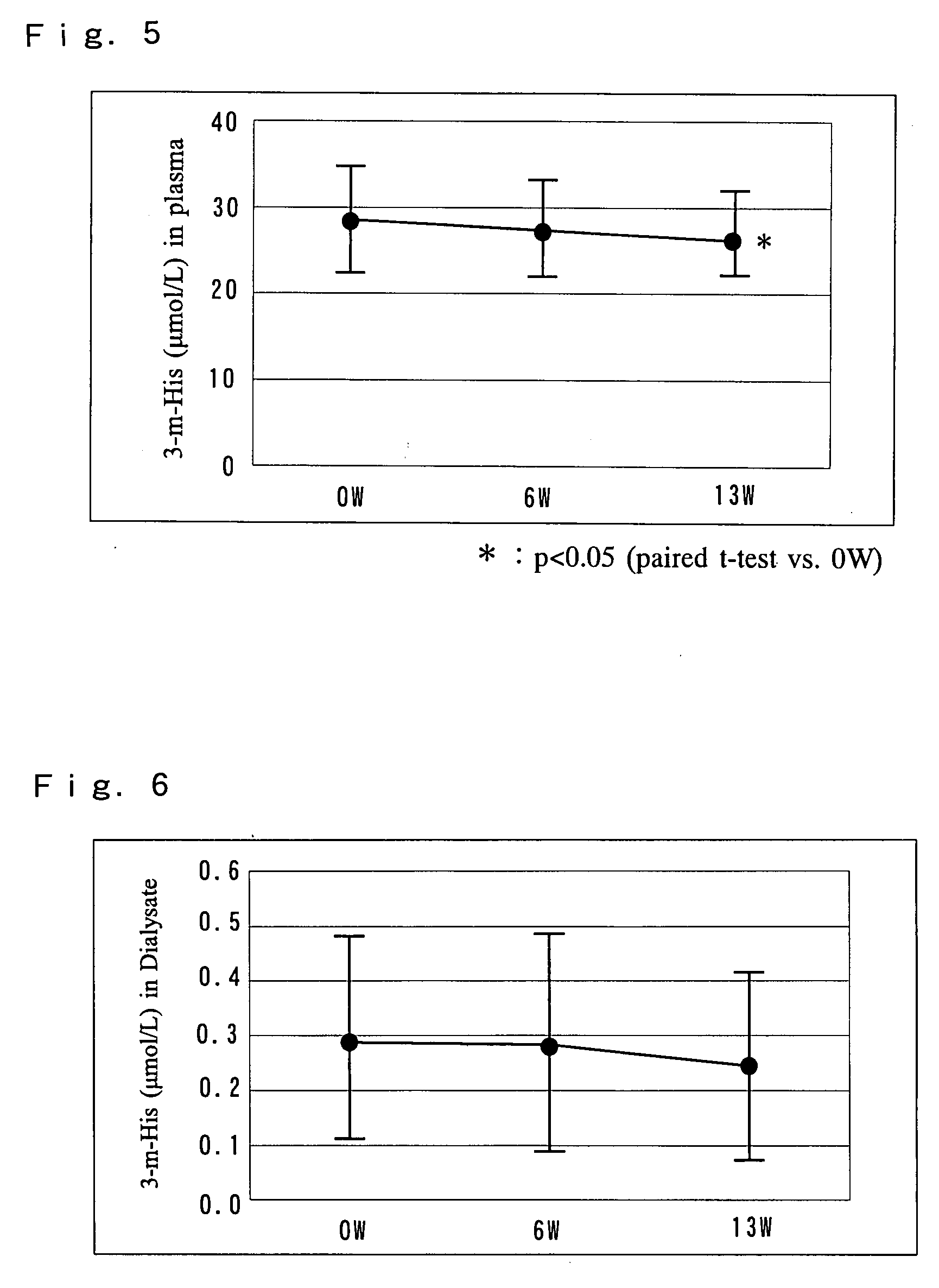
A pharmaceutical preparation including an amino acid infusion fluid is administered to patients receiving dialysis to ameliorate nutritional status of patients. As a result, anemia can be ameliorated and the required dose of erythropoietin can be reduced in these patients. Furthermore, the serum phosphate levels can be controlled to a predetermined range and protein catabolism can be suppressed in such patients. The amino acid infusion fluid has at least one essential amino acid. The preferred amino acid composition includes at least L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-threonine, L-tryptophan, L-valine, L-alanine, L-arginine, L-aspartic acid, L-glutamic acid, L-histidine, L-proline, L-serine, L-tyrosine, glycine and L-cysteine, wherein the ratio of essential amino acids to non-essential amino acids is 2.5 or higher.
Owner:AJINOMOTO CO INC
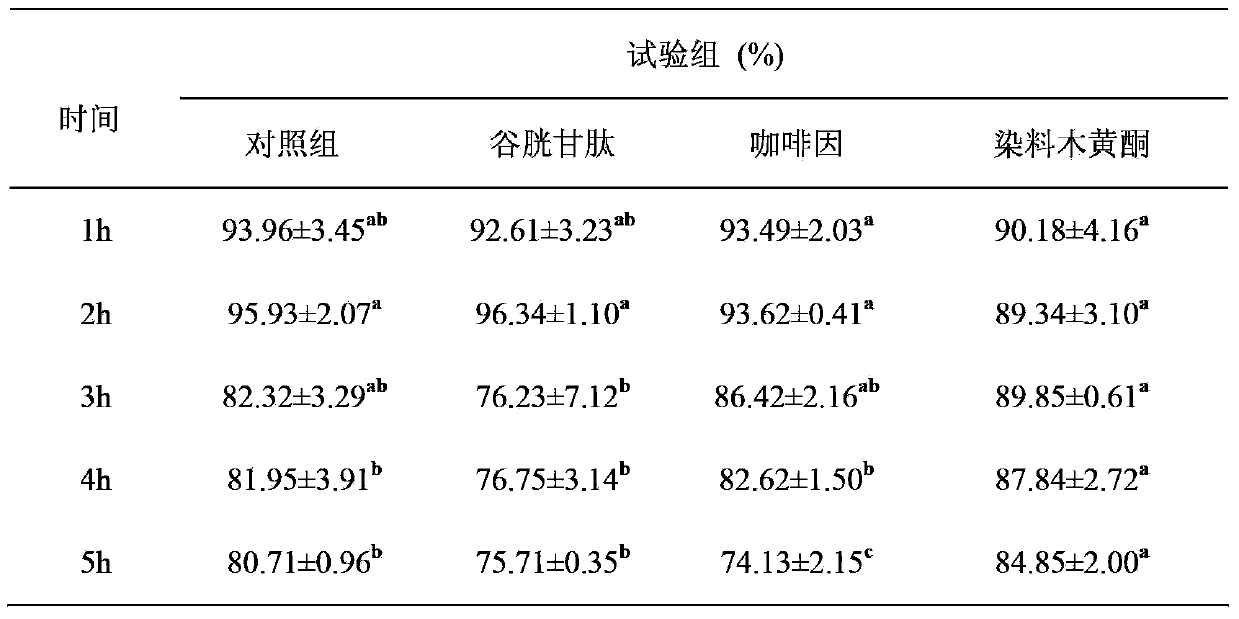
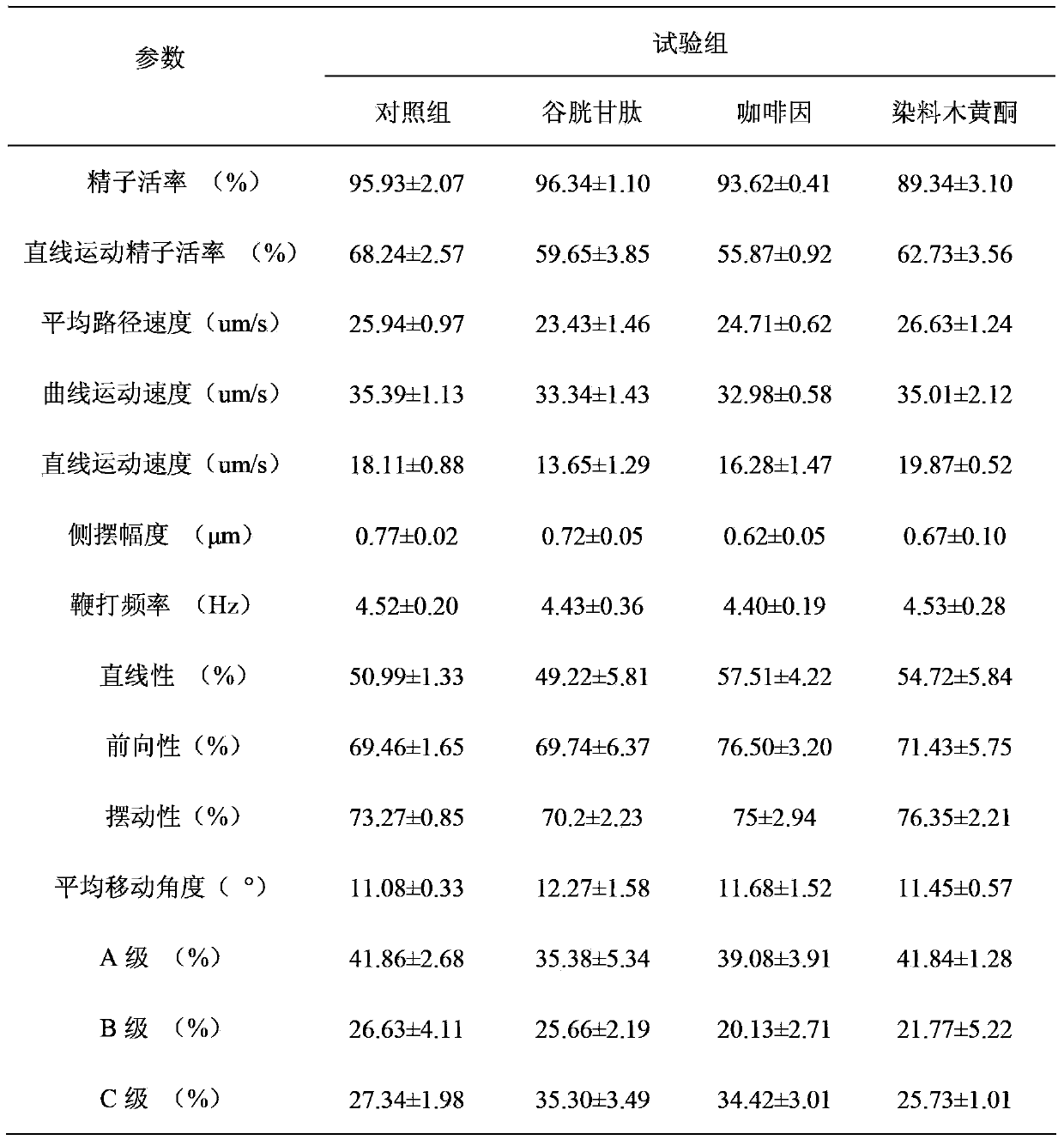
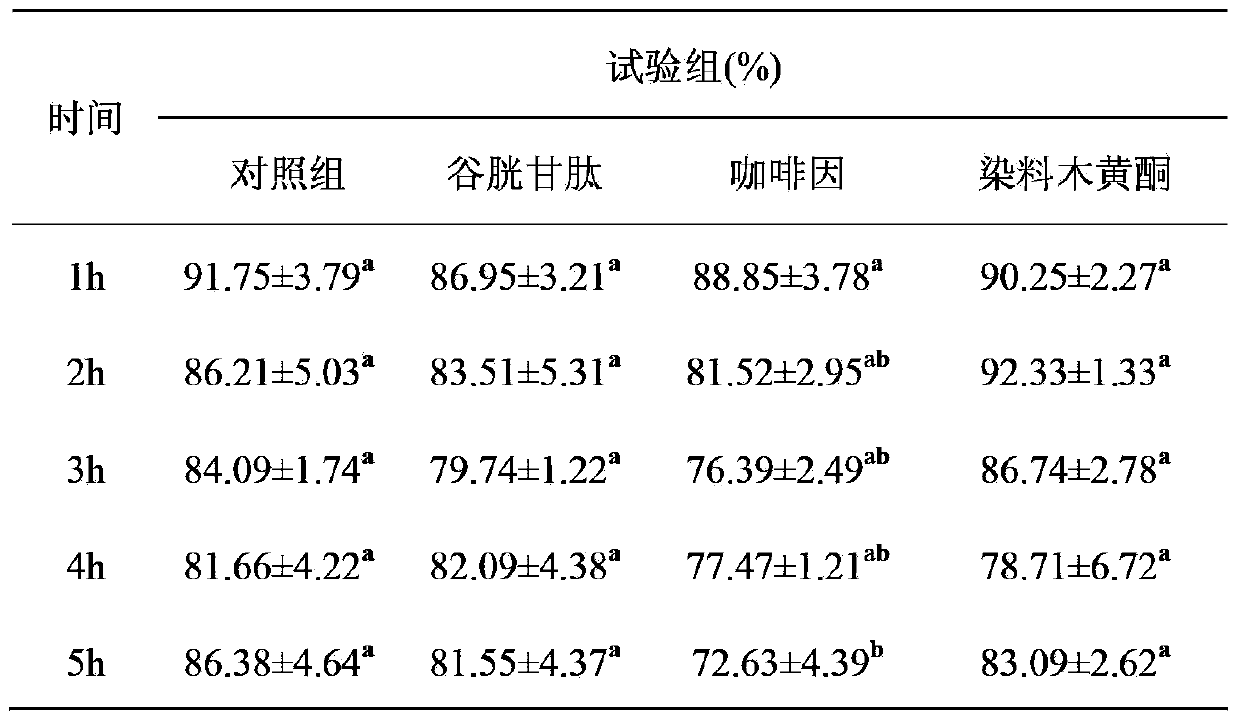
Method for improving sperm motility and increasing in-vitro-fertilization rate of frozen bovine semen by aid of genistein
ActiveCN103462724AEfficient removalReduces Antioxidative DamageAnimal reproductionSerum phosphateBottle
The invention provides a method for improving the sperm motility and increasing an in-vitro-fertilization rate of frozen bovine semen by the aid of genistein. The method is particularly characterized in that the genistein containing 1%o of DMSO (dimethyl sulfoxide) is added into SP-TALP (serum phosphate-total alkaline phosphatase) washing liquid for washing the frozen bovine semen in earlier-stage processing for the frozen bovine semen, or mixed liquid of PHE (phenylalanine) capacitation liquid and IVF-TALP (in vitro fertilization-total alkaline phosphatase) fertilization liquid containing bovine semen in a capacitation processing stage of second-stage pre-fertilization semen or prepared fertilization drops containing oocytes in a third-stage in-vitro-fertilization procedure; the added amounts of the genistein in two earlier stages of a fertilization procedure of each bottle of frozen bovine semen are 10 micromoles per liter, and the final concentration of the genistein in mixed liquid of the bovine semen and the fertilization drops containing the oocytes is 10 micromoles per liter when the genistein is added in a third stage of the fertilization procedure of each bottle of frozen bovine semen. The method has the advantages that the genistein is respectively used in the three in-vitro-fertilization stages, so that the sperm motility is greatly improved, and the sperm fertilization rate is greatly increased.
Owner:武汉市畜牧兽医科学研究所
Method of reducing phosphate absorption by administering orally an IgY anti-intestinal sodium phosphate cotransporter type 2B antibody
ActiveUS8802094B2Eliminate side effectsReduce hyperphosphatemiaMetabolism disorderImmunoglobulins against cell receptors/antigens/surface-determinantsSerum phosphateAntiendomysial antibodies
A method for reducing phosphate absorption in a human or non-human animal subject at risk of developing or having developed hyperphosphatemia is disclosed. The method includes the step of administering orally to the subject an anti-intestinal sodium phosphate cotransporter type 2B (Npt2B) antibody in an amount effective to reduce or maintain the serum phosphate concentration in the subject.
Owner:CYCTOCHROMA INC +1
Health-care granules for preventing negative balance of energy metabolism of cows and using method of health-care granules
InactiveCN105494959AEasy to useImprove palatabilityAnimal feeding stuffAccessory food factorsDiseaseFood consumption
The invention discloses health-care granules for preventing the negative balance of energy metabolism of cows and a using method of the health-care granules. The health-care granules disclosed by the invention are mainly prepared from the following raw materials: calcium propionate, brown sugar, magnesium chloride, yeast, sodium chloride and corn flour. The health-care granules for feeding the cows, disclosed by the invention, have the advantages of being convenient to use, low in cost, good in palatability, notable in effects and the like. After the postnatal cows are fed with the health-care granules, physical strength can be quickly restored, the quantity of rumen microbial populations can be maintained, the food consumption is restored, the milk yield is increased, and the ability of resisting diseases is improved. Feeding experiments for the postnatal cows confirm that the health-care granules can effectively maintain the level of blood calcium (Ga), serum phosphate (P), blood magnesium (Mg) and blood sugar (GLU), and can reduce the level of beta-hydroxybutyric acid (BHBA), free fatty acid (NEFA), and aspartate aminotransferase (AST). The attack rate of nutrient metabolic disease-ketosis of the postnatal cows can be effectively reduced.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Methods and compositions for phosphate binding
ActiveUS20090004133A1Avoid absorptionReducing intestinal phosphate absorptionOrganic active ingredientsOrganic chemistrySerum phosphateHydrobromide
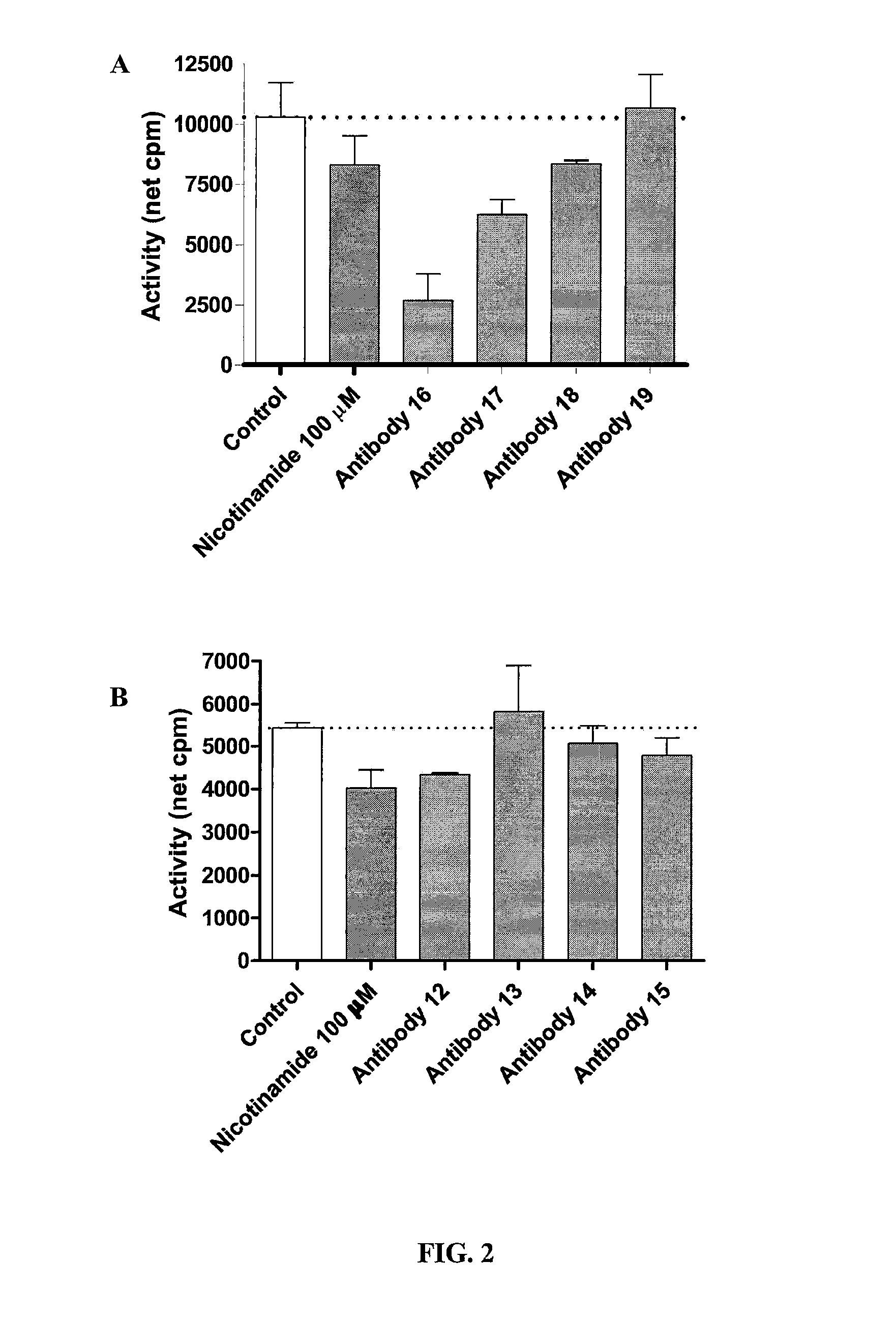
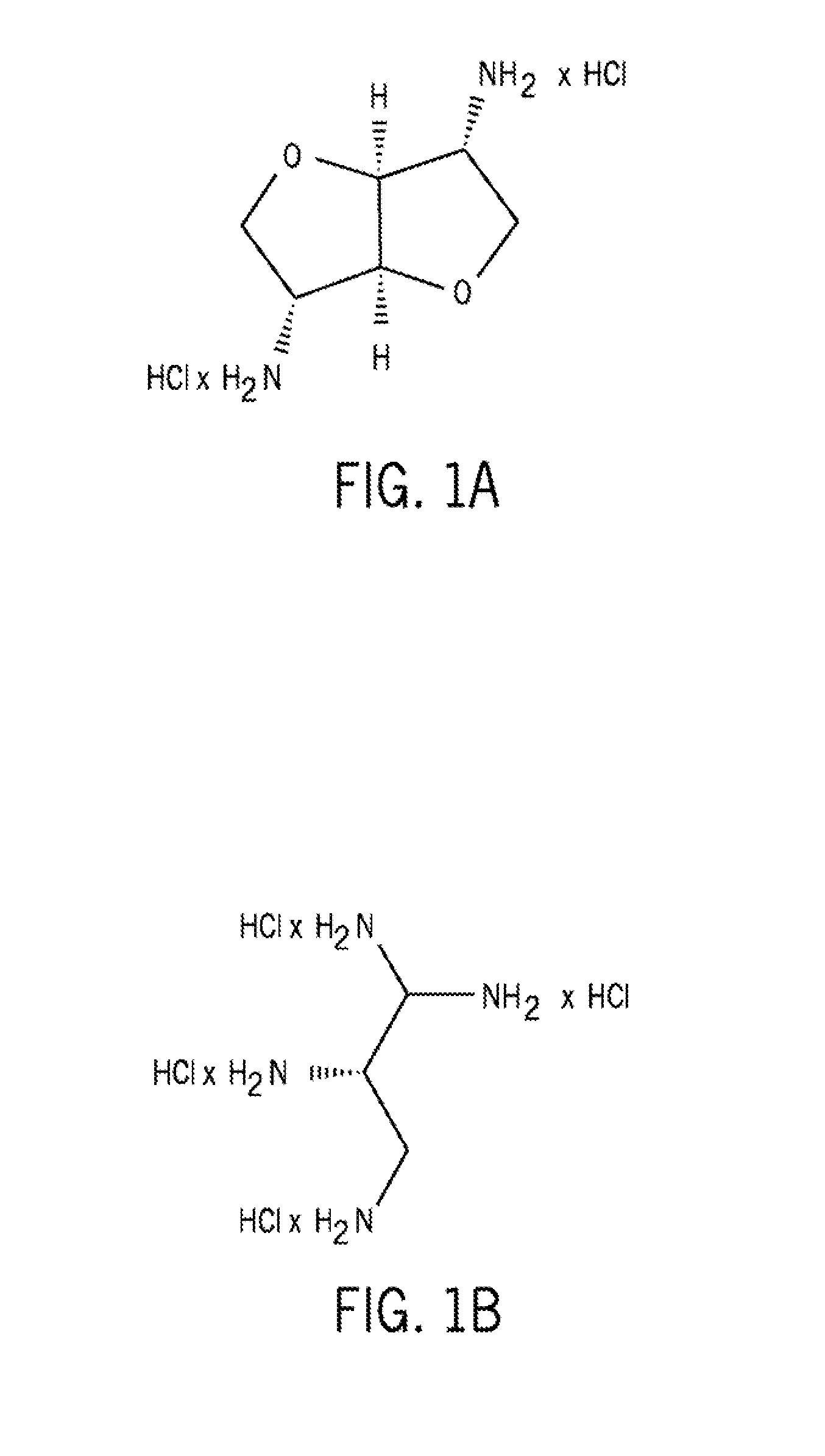
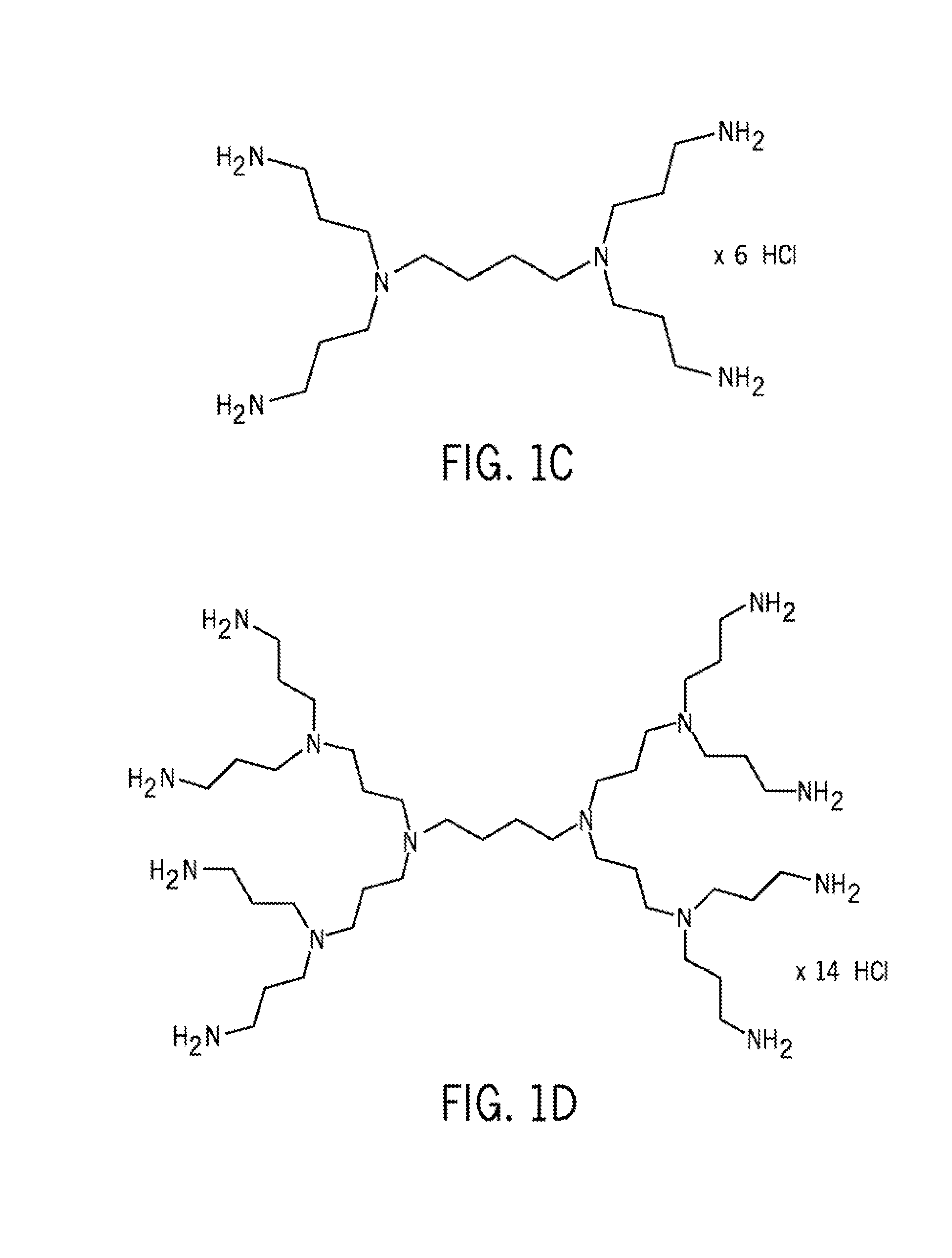
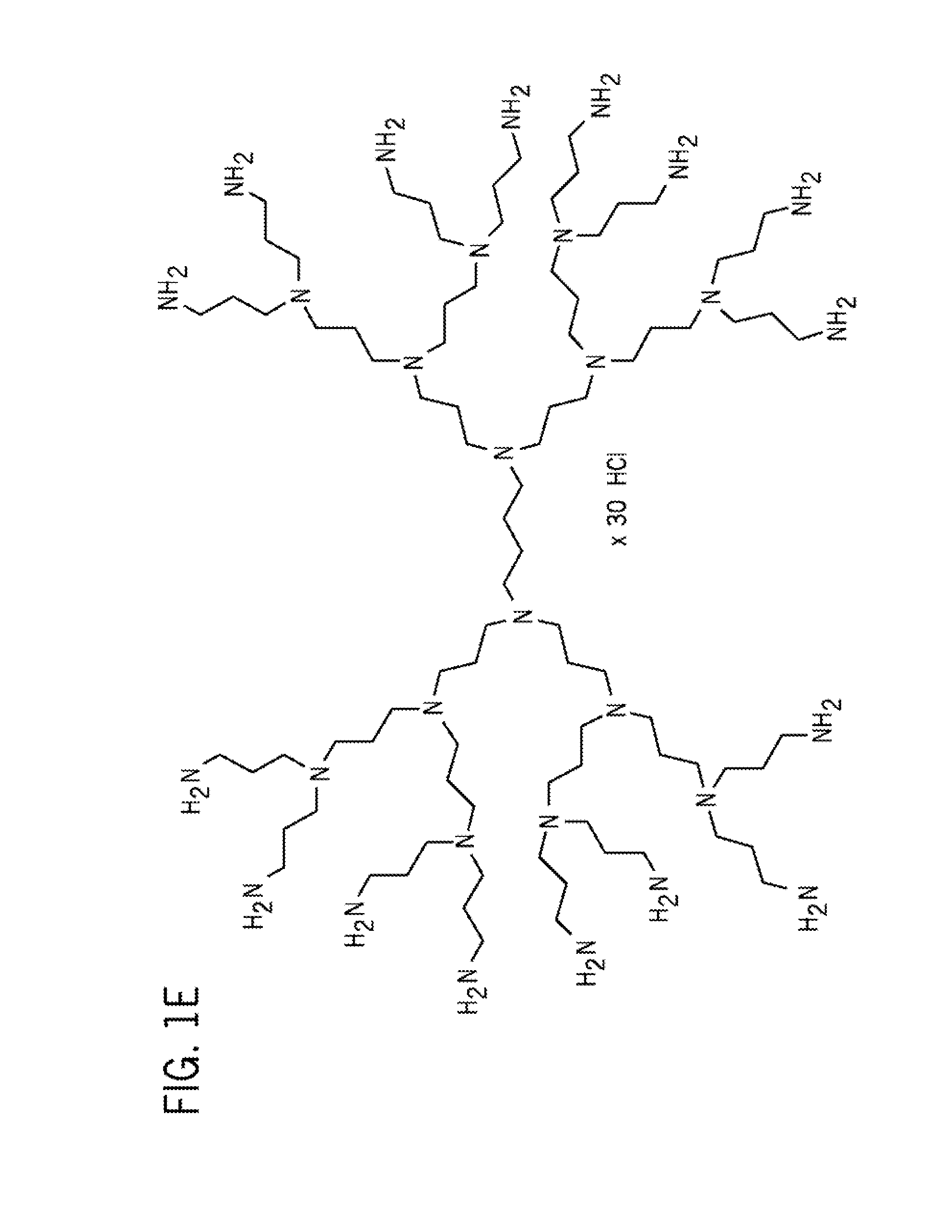
The present invention provides improved methods and compositions for therapeutically controlling and / or reducing serum phosphate levels in animals and mammalian patients. The methods comprise administering to the patient an amount of a dendrimer composition effective to prevent absorption of substantial amounts of phosphate from the patient's GI tract. In a preferred version, a dose of between 2.5 and 15 grams per day is effective to prevent over 80% of phosphate present in the patient's GI tract from being absorbed. The dendrimer composition may comprise a hydrocitrate, hydrochloride, hydrobromide, hydroacetate or hydroanionic form.
Owner:WISCONSIN ALUMNI RES FOUND
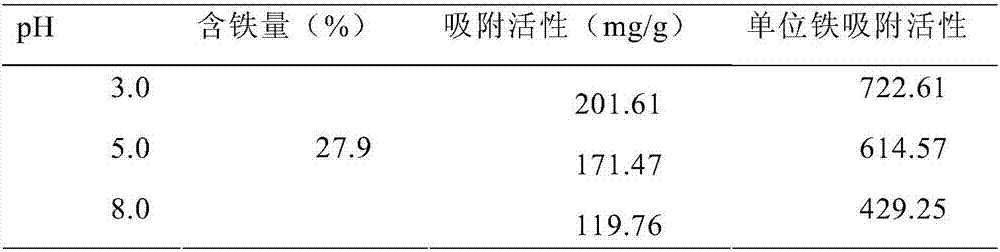
Iron-based hydroxide-low molecular weight sugar phosphate binder as well as preparation method and application thereof
ActiveCN107397760AHeavy metal active ingredientsOrganic active ingredientsSerum phosphateSugar phosphates
The invention provides an iron-based hydroxide-low molecular weight sugar phosphate binder as well as a preparation method and application thereof. Specifically, the invention provides a phosphate binder which comprises an iron-based hydroxide and low molecular weight sugar, wherein the number of monosaccharide units of the low molecular weight sugar is 3-20, and based on the total weight of the phosphate binder, the mass ratio of the iron is 2-45wt%. Experiments prove that the phosphate binder has an obvious effect of lowering the serum phosphate level. Therefore, the phosphate binder has wide application prospects.
Owner:CINKATE PHARMA INTERMEDIATES
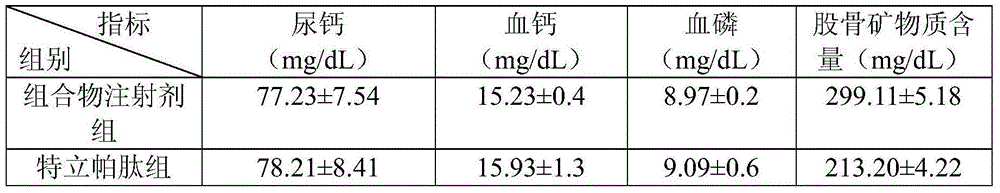
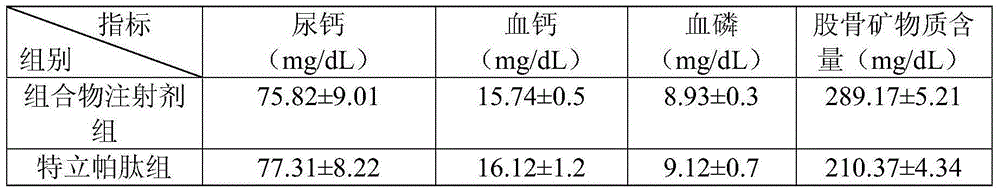
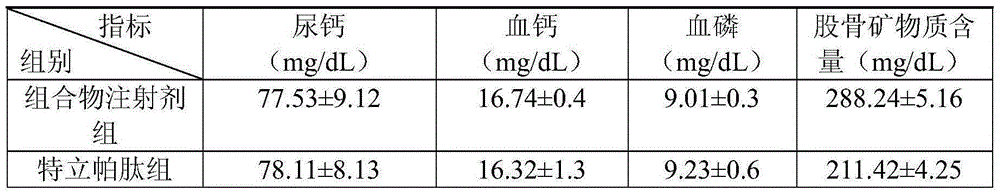
Medicine composite for treating osteoporosis and preparation method thereof
InactiveCN105169394AGood treatment effectReduced responseOrganic active ingredientsPeptide/protein ingredientsSerum phosphateTreatment effect
The invention relates to a medicine composite injection for treating osteoporosis and a preparation method thereof. In particular, active constituents of the injection comprise one or more constituents out of teriparatide combined with bisphosphonate series, calcitonin series and estrogen, and besides, pH regulating agents and excipient with the amount acceptable to pharmacy are added. According to the preparation method, the preparation process is investigated, the rat urinary calcium content, the blood calcium conent, the serum phosphate content and the thighbone mineral matter content further serve as indexes, the treatment effect of the injection on osteoporosis model rats is further investigated, the prepared composite injection is remarkable in effect, and the practical significance is achieved.
Owner:SHENZHEN JYMED TECH
Methods and compositions for phosphate binding
ActiveUS8114393B2Avoid absorptionReducing intestinal phosphate absorptionOrganic active ingredientsOrganic chemistrySerum phosphateHydrobromide
Owner:WISCONSIN ALUMNI RES FOUND
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
InactiveUS20190269722A1Long-term administrationImprove scalabilityOrganic active ingredientsBiocideSerum phosphatePhosphate level
The present invention discloses pharmaceutical-grade ferric organic compounds, including ferric citrate, which are soluble over a wider range of pH, and which have a large active surface area. A manufacturing and quality control process for making a pharmaceutical-grade ferric citrate that consistently complies with the established Manufacture Release Specification is also disclosed. The pharmaceutical-grade ferric organic compounds are suitable for treating disorders characterized by elevated serum phosphate levels.
Owner:PANION & BF BIOTECH INC
Iron-based hydroxide-degraded carbohydrate phosphate binder as well as preparation method and application thereof
The invention provides an iron-based hydroxide-degraded carbohydrate phosphate binder as well as a preparation method and application thereof. Specifically, the invention provides a phosphate binder which comprises an iron-based hydroxide and a degraded carbohydrate, wherein based on the total weight of the phosphate binder, the mass ratio of the iron is 5-45wt%; the number-average molecular weight of the degraded carbohydrate is 1000-300000Da; and a ratio (D1 / D2) of the number-average molecular weight D1 of the degraded carbohydrate to a number-average molecular weight D2 of the carbohydrate before degradation is 0.05-0.8. Experiments prove that the phosphate binder has an obvious effect of lowering the serum phosphate level. Therefore, the phosphate binder has wide application prospects.
Owner:CINKATE PHARMA INTERMEDIATES
Methods for reducing phosphate absorption
InactiveUS20100143385A1Reducing phosphate absorptionReduce and maintain serum phosphate concentrationAntibacterial agentsEgg immunoglobulinsSerum phosphateHuman animal
It is disclosed here a method for reducing phosphate absorption in a human or non-human animal subject wherein the subject consumes a diet containing phytic acid or phytate and either has or is at risk of developing hyperphosphatemia. The method includes the step of administering orally to the subject an anti-phytic acid (C6H6[OPO(OH)2]o) antibody or an anti-phytate antibody in an amount effective to reduce or maintain the serum phosphate concentration in the subject.
Owner:CYCTOCHROMA INC +1
A nanocomposite material for adsorbing phosphorus and its preparation and application
ActiveCN107412166BImprove adsorption capacityNot easy to catchOrganic active ingredientsMetabolism disorderSerum phosphateSevelamer
The invention relates to a phosphate adsorption composite nanomaterial as well as preparation and an application thereof. The composite nanomaterial comprises a nano-carrier and Sevelamer carbonate in the mass ratio being (3-12):1, wherein the nano-carrier comprises lipidosome, and Sevelamer carbonate is polymer carbonate of 2-propen-1-amine and epoxy chloropropane. The phosphate adsorption nano-composite material is used for preparing a medicine for reducing serum phosphate. Compared with the prior art, the excellent adsorption performance of polymer carbonate of 2-propen-1-amine and epoxy chloropropane is sufficiently utilized, the advantages that the long-circulation nano-carrier is non-toxic, stable and not easily captured by a phagocytic system in a body are played, and the high-biocompatibility lipidosome / Sevelamer composite nanomaterial easy to excrete is prepared. The method adopts a simple preparation process and easily controllable conditions and is thus suitable for clinic treatment of intractable hyperphosphatemia.
Owner:TONGJI UNIV
Methods and compositions for phosphate binding
ActiveUS8158117B2Avoid absorptionReducing intestinal phosphate absorptionBiocideNanotechHydrobromideSerum phosphate
The present invention provides improved methods and compositions for therapeutically controlling and / or reducing serum phosphate levels in animals and mammalian patients. The methods comprise administering to the patient an amount of a dendrimer composition effective to prevent absorption of substantial amounts of phosphate from the patient's GI tract. In a preferred version, a dose of between 2.5 and 15 grams per day is effective to prevent over 80% of phosphate present in the patient's GI tract from being absorbed. The dendrimer composition may comprise a hydrochloride, hydrobromide, hydroacetate or hydroanionic form.
Owner:WISCONSIN ALUMNI RES FOUND
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
PendingUS20210299168A1Improve scalabilityImprove the preparation effectOrganic active ingredientsHeavy metal active ingredientsSerum phosphateFerric citrate
Owner:PANION & BF BIOTECH INC
Phosphate adsorption composite nanomaterial as well as preparation and application thereof
ActiveCN107412166AImprove adsorption capacityEasy to catchOrganic active ingredientsMetabolism disorderSerum phosphateEpoxy
The invention relates to a phosphate adsorption composite nanomaterial as well as preparation and an application thereof. The composite nanomaterial comprises a nano-carrier and Sevelamer carbonate in the mass ratio being (3-12):1, wherein the nano-carrier comprises lipidosome, and Sevelamer carbonate is polymer carbonate of 2-propen-1-amine and epoxy chloropropane. The phosphate adsorption nano-composite material is used for preparing a medicine for reducing serum phosphate. Compared with the prior art, the excellent adsorption performance of polymer carbonate of 2-propen-1-amine and epoxy chloropropane is sufficiently utilized, the advantages that the long-circulation nano-carrier is non-toxic, stable and not easily captured by a phagocytic system in a body are played, and the high-biocompatibility lipidosome / Sevelamer composite nanomaterial easy to excrete is prepared. The method adopts a simple preparation process and easily controllable conditions and is thus suitable for clinic treatment of intractable hyperphosphatemia.
Owner:TONGJI UNIV
FGF23 c-tail fusion proteins
Owner:PFIZER INC +1
Chinese medicine for treating fracture and preparation method thereof
ActiveCN101474391APromote hyperplasiaPromote healingHeavy metal active ingredientsSkeletal disorderDegenerative changeMyrrh
The invention discloses a medicament for treating fracture and a preparation method thereof. The medicament is prepared by traditional Chinese medicine raw materials such as rhizome drynariae, pyrite, corydalis tuber, notoginseng, turmeric, libanotus, myrrh, dragon's blood, sandalwood, dalbergia wood and eaglewood. The medicament can tonify the kidney and strengthen bone, rejoin muscle and ligament, relieve bruises, relieve injury and stop pain, relax tendons, invigorate blood circulation, promote the bone to absorb calcium and improve levels of blood calcium and serum phosphate, is favorable for calcification and sclerotin formation of the bone, and can also improve the function of chondrocyte, postpone degenerative change of cells, reduce pathological change rate of arthrosis, and promote proliferation of netlike cells and haemoglobin in the peripheral blood of the bone marrow; and compared with the various existing orthopedics medicaments, the medicament can better promote poroma proliferation and accelerate fracture healing, has no toxic side effects, can be used by patients from any age group, has no adverse reaction report by now, and has superior curative effect.
Owner:吉学忠
Blood phosphorus adsorbent and preparation method thereof, adsorption column for hemoperfusion
ActiveCN104258829BRemissionRelieve painOther chemical processesOther blood circulation devicesSerum phosphateCellulose
The invention provides a serum phosphate adsorbing agent; a hydrophilic carrier is immobilized by ligands capable of capturing phosphate ions to obtain the serum phosphate adsorbing agent; the hydrophilic carrier is selected from one or more out of cellulose, agarose and polyvinyl alcohol microsphere, and the ligands capable of capturing phosphate ions refer to amino polymers. The invention also provides a preparation method of the serum phosphate adsorbing agent and an adsorption column for blood perfusion, prepared by the serum phosphate adsorbing agent. Compared with medicines which are orally taken to act on the human body, the serum phosphate adsorbing agent can directly remove the phosphate ions in blood through blood circulation, so as to immediately alleviate the illness state of a patient; and the serum phosphate adsorbing agent can be combined with hemodialysis, oral taking pain of the patient each day can be reduced, and the result caused by a situation that the patient forgets to take the medicine can be avoided.
Owner:JAFRON BIOMEDICAL
Methods of reducing phosphate absorption
ActiveUS20100233184A1Attenuate and prevent hyperphosphatemiaAntibacterial agentsEgg immunoglobulinsSerum phosphateHuman animal
Owner:WISCONSIN ALUMNI RES FOUND +1
Once A Day Formulation for Phosphate Binders
InactiveUS20090304623A1Improve complianceImprove effectivenessOrganic active ingredientsHeavy metal active ingredientsSerum phosphateCalcium Binder
A method for reducing serum phosphate in a subject in need thereof comprising administering once per day to said subject a phosphate binder, wherein the phosphate binder has a phosphate binding capacity of at least 52 mmole.
Owner:GENZYME CORP
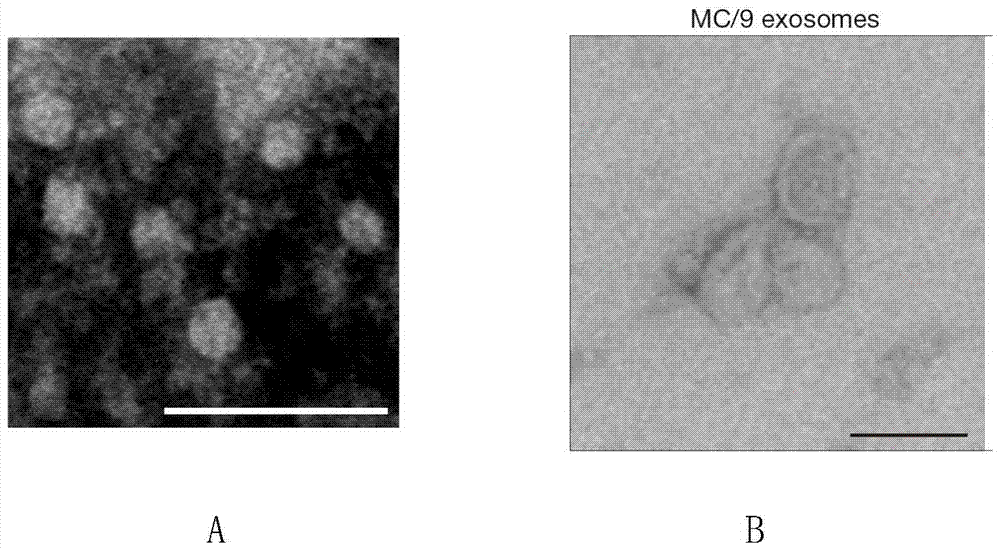
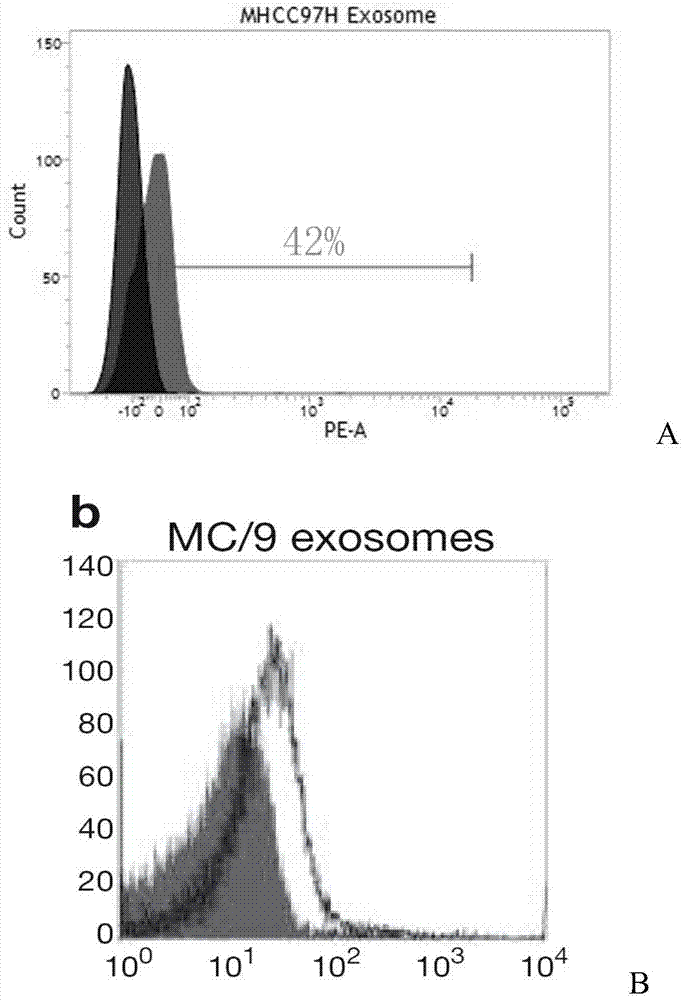
A method for rapid isolation of exosomes
The invention relates to a method for rapidly separating exosomes from cells. The method comprises: collecting the living cell culture supernatant, centrifuging to remove cell debris, collecting the supernatant and ultrafiltration for 10-15 minutes in an ultrafiltration tube with a molecular weight cut-off of 100KD, collecting the supernatant to obtain a concentrated solution, adding the concentrated solution to volume In a pH9-12 phosphate buffer solution containing 0.1-0.2% (v / v) fetal bovine serum that is 4-10 times the volume of the concentrated solution, ultracentrifuge at 140000-150000g and 4°C for 70-90min, take the precipitate, and weigh The exosomes are obtained by suspending in phosphate buffer solution with pH 9-12. The beneficial effects of the present invention are mainly reflected in: 1. The separation time is short. 2. High yield. 3. High purity.
Owner:FUZHOU HOSPITAL FOR INFECTIOUS DISEASE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com