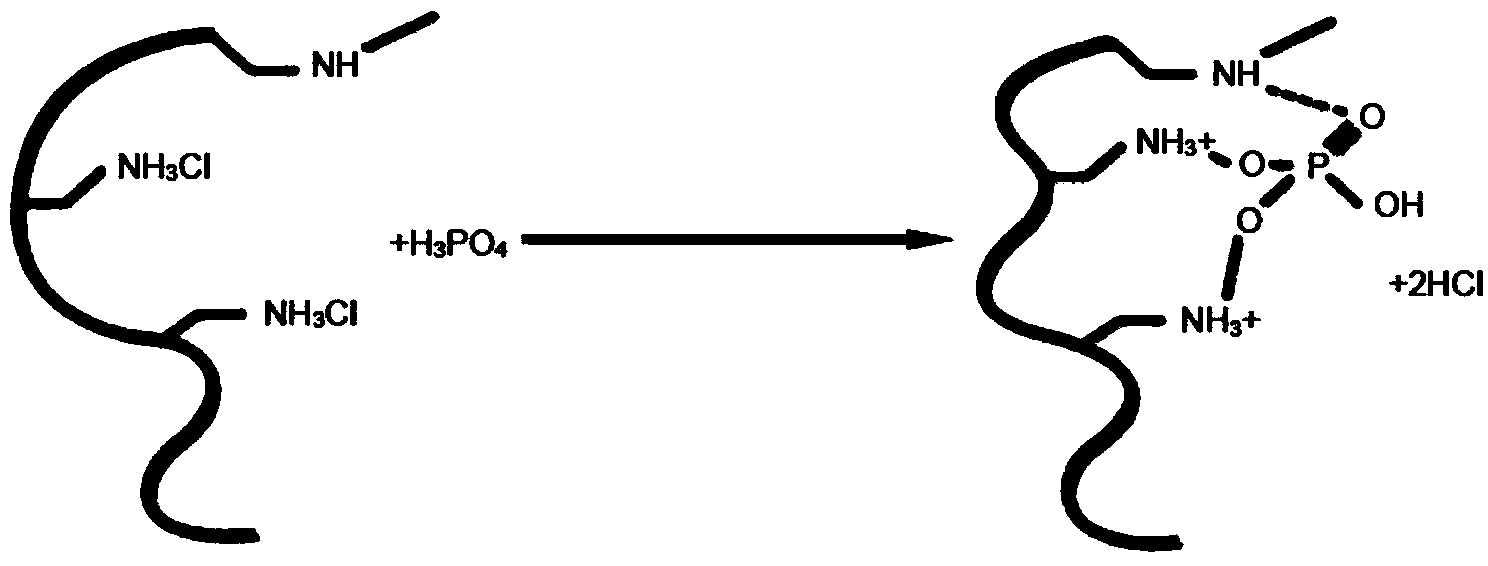
Serum phosphate adsorbing agent and preparation method thereof and adsorption column for blood perfusion
An adsorbent and blood phosphorus technology, applied in the medical field, can solve the problem of inability to directly remove phosphorus, and achieve the effect of reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
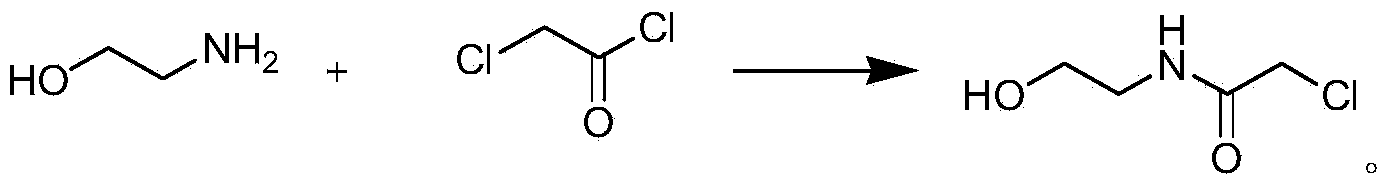
[0044] 1. Synthesis of Hydroxyethylacetamide
[0045] Add 40g of toluene to a three-neck flask equipped with a constant pressure funnel and a stirrer, add 1g of ethanolamine, 3g of 10wt% sodium carbonate solution, add 2g of chloroacetyl chloride dropwise at 20-30°C, and keep stirring at 30°C for 3h. Wherein toluene is a solvent, and ethanolamine and sodium carbonate solution are reaction accelerators. The sodium carbonate solution in the present embodiment can also be replaced with solutions such as sodium bicarbonate, potassium bicarbonate, potassium carbonate. After the reaction is completed, stir and cool, filter, and dry to obtain the product glycolic acid amide:
[0046]
[0047] 2. Carrier grafting reaction
[0048] Add 5g of anhydrous potassium carbonate to a 100mL three-necked flask, then add 50g of N,N-dimethylformamide and 10g of acetone mixed solution, then add 1g of agarose microspheres and 0.05g of tetrabutylammonium bromide, 2g steps The hydroxyethylacetami...
Embodiment 2
[0061] 1. Synthesis of Hydroxyethylacetamide
[0062] Add 100g of toluene to a three-necked flask equipped with a constant pressure funnel and a stirrer, add 8g of ethanolamine, 7g of 10wt% sodium carbonate solution, drop 18g of chloroacetyl chloride at 20-30°C, and keep stirring at 30°C for 6h. After the reaction is completed, stir and cool, filter, and dry to obtain the product glycolic acid amide:
[0063]
[0064] 2. Carrier grafting reaction
[0065] Add 25g of anhydrous potassium carbonate to a 250mL three-necked flask, then add 90g of N,N-dimethylformamide and 50g of acetone mixed solution, then add 5g of cellulose microspheres and 0.15g of tetrabutylammonium bromide, 5g steps The hydroxyethylacetamide synthesized in 1 was reacted at room temperature for 24 hours. After the reaction was completed, the acetone was distilled off, washed with water and saturated saline, and N,N-dimethylformamide was washed with ethanol:
[0066]
[0067] S is a hydrophilic carrier,...
Embodiment 3
[0077] 1. Synthesis of Hydroxyethylacetamide
[0078] Add 60g of toluene to a three-necked flask equipped with a constant pressure funnel and a stirrer, add 3g of ethanolamine, 3g of 10wt% sodium carbonate solution, drop 8g of chloroacetyl chloride at 20-30°C, and keep stirring at 30°C for 4h. After the reaction is completed, stir and cool, filter, and dry to obtain the product glycolic acid amide:
[0079]
[0080] 2. Carrier grafting reaction
[0081] Add 10g of anhydrous potassium carbonate to a 250mL three-necked flask, then add 60g of N,N-dimethylformamide and 30g of acetone mixed solution, then add 3g of polyvinyl alcohol microspheres and 0.1g of tetrabutylammonium bromide, 3g The hydroxyethylacetamide synthesized in step 1 was reacted at room temperature for 16 hours. After the reaction was completed, the acetone was distilled off, washed with water and saturated saline, and N,N-dimethylformamide was washed with ethanol:
[0082]
[0083] S is a hydrophilic carr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com