Ferric organic compounds, uses thereof and methods of making same
A pharmaceutical-grade compound technology, applied in the field of pharmaceutical-grade iron-organic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
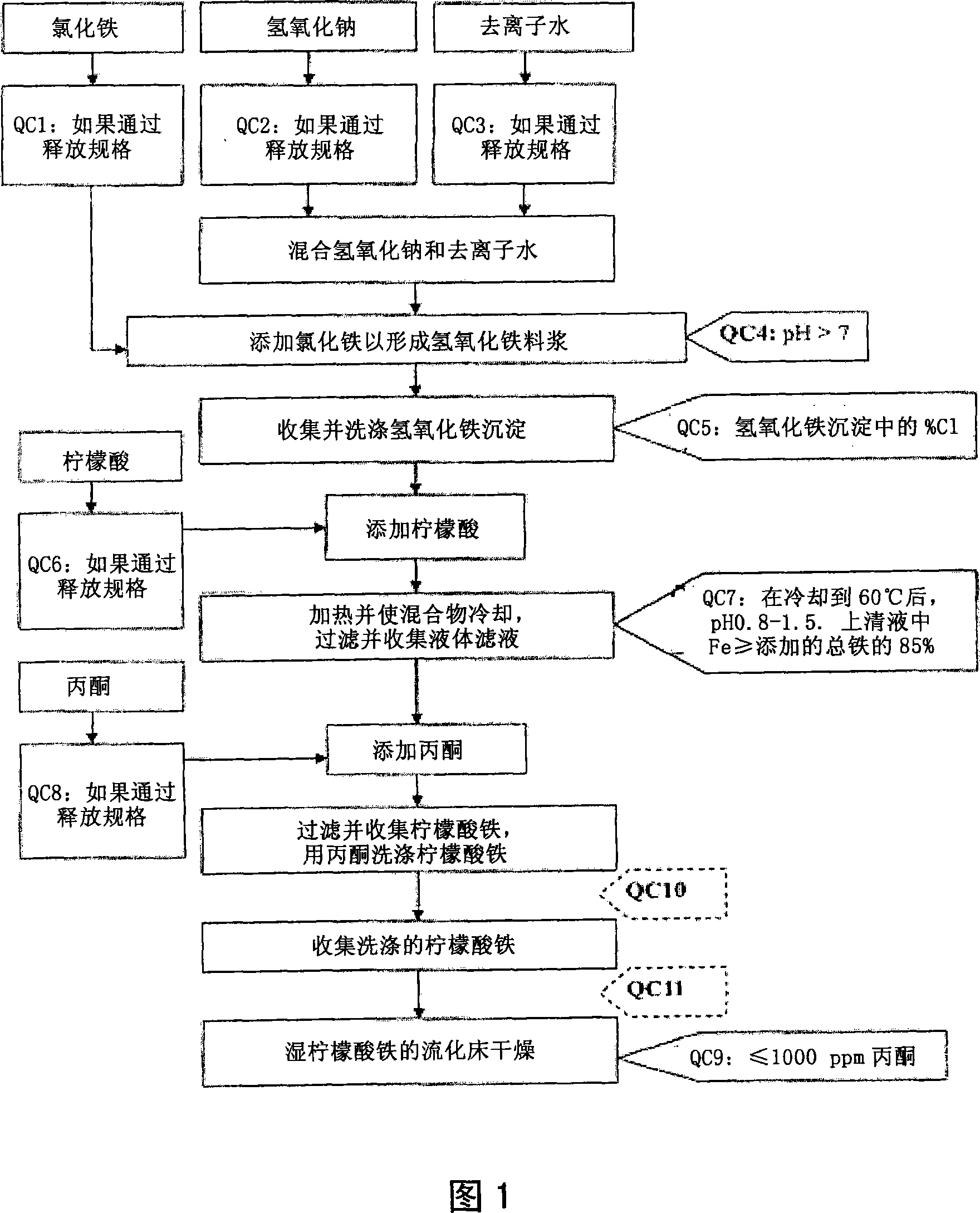
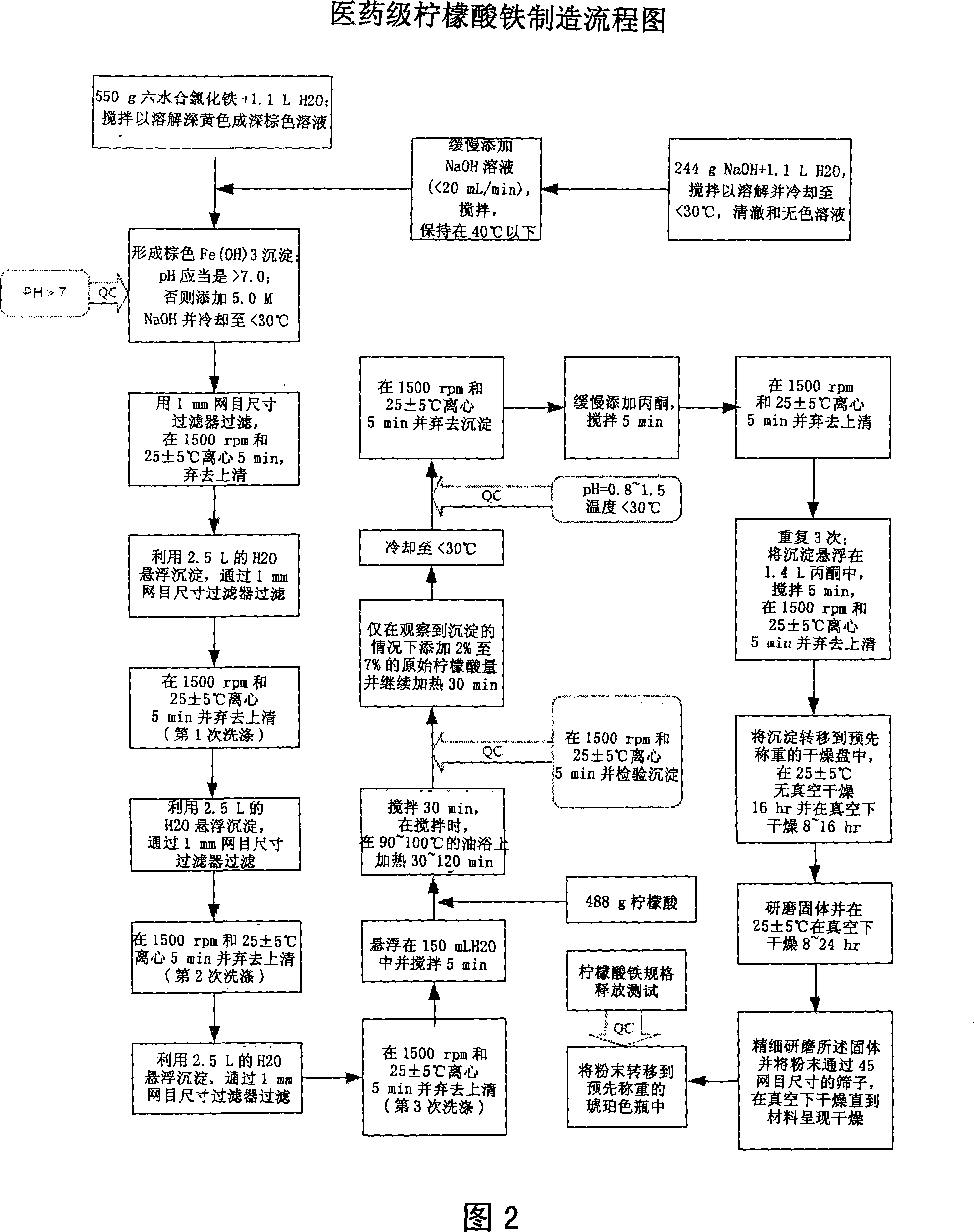
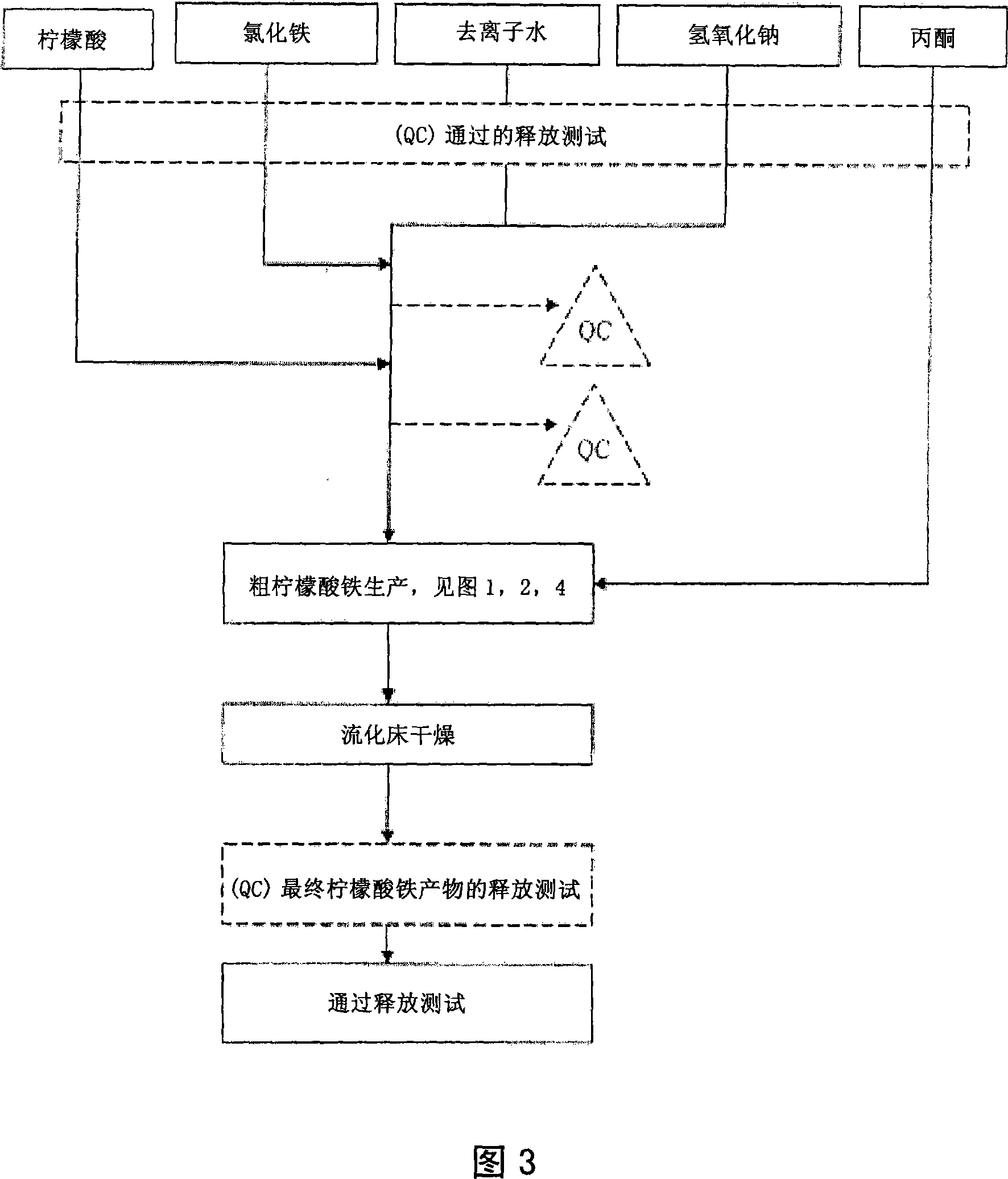
[0138] Example 1. General method for the synthesis of pharmaceutical grade ferric citrate
[0139] With respect to Fig. 1, which shows the general method for synthesizing pharmaceutical grade ferric citrate according to the present invention, the flow chart shows the instrumentation of quality control measures at selected stages of the synthetic process to ensure that the final ferric citrate product complies with those shown in Table A or any established manufacturing release specification for pharmaceutical grade ferric citrate that is approved or suitable for human use. Other quality control measures or steps, apparent to those of ordinary skill in the art, can be used or incorporated into pharmaceutical grade synthesis processes to maintain the quality and purity of the final product and to increase the efficiency and yield of the synthesis process. See, eg, QC10 and QC11 in FIG. 1 .
[0140]Examples of quality control measures employed during synthesis include: (QC4) kee...
Embodiment 1A
[0142] Example 1A. General method for the synthesis of pharmaceutical grade iron organic compounds
[0143] With respect to Figure 10, which shows the general method for the synthesis of pharmaceutical grade ferric organic compounds according to the present invention, the flow diagram shows the implementation of quality control measures at selected stages of the synthetic process to ensure that the final ferric citrate product complies with the requirements for the approved Pharmaceutical-grade iron-organic compounds that are or are suitable for human use have established manufacturing release specifications. Other quality control measures or steps, apparent to those of ordinary skill in the art, can be used or incorporated into pharmaceutical grade synthesis processes to maintain the quality and purity of the final product and to increase the efficiency and yield of the synthesis process. See, eg, QC10A and QC11A in FIG. 10 .
[0144] Examples of quality control measures emp...
Embodiment 2
[0146] Example 2. Solubility properties of iron organic compounds according to the invention
[0147] Ferric organic compounds produced according to the method described above are more soluble over a wider range of pH levels than commercially available ferric organic compounds. The increased solubility of the inventive ferric organic compounds is believed to be a result of the uniquely large active surface area of the inventive ferric organic compounds. For example, at pH 8.0, the intrinsic dissolution rate of ferric citrate of the present invention is 3.08 times greater than that of commercially available ferric citrate. See Table 1.
[0148] The intrinsic dissolution rate of commercially available ferric citrate was compared with that of the present invention. The intrinsic dissolution rate is specified as the dissolution rate of the pure substance under constant surface area conditions. The dissolution rate and bioavailability of a drug substance are influenced by its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com