Medicine composite for treating osteoporosis and preparation method thereof
A technology for osteoporosis and composition, which is applied in the field of pharmaceutical preparations and can solve the problems of lack of response and increase of bone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Teriparatide 600mg, alendronate sodium 300g, mannitol 300g, appropriate amount of hydrochloric acid buffer.
[0022] The preparation process is as follows: accurately weigh the main drug in the prescribed amount, add appropriate amount of water for injection to dissolve; add the prescribed amount of mannitol and hydrochloric acid buffer to the above solution in turn, and adjust the pH to 7.0; Filter through a microporous membrane, set the volume to 2400ml, pack into 1000 tubes, press the plug, take out the box, tie the cover, and after passing the inspection, label and pack to obtain the composition injection.
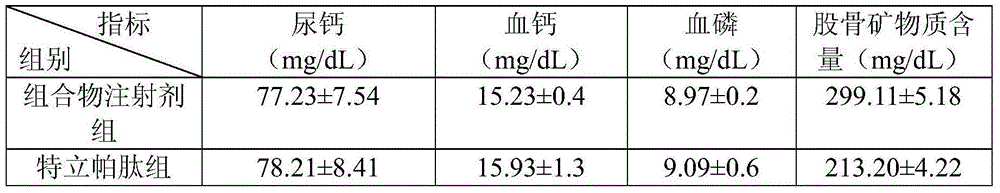
[0023] Therapeutic effect of the composition injection prepared in Example 1 on the osteoporosis rat model.
[0024] Experimental animals: 40 SD female rats, weighing about 200g, used 1% cellulose solution to add retinoic acid to adjust to an appropriate concentration, and administered 80mg / kg / d for 20 days, once a day, to form osteoporosis rats Model.
[0025...
Embodiment 2
[0030] Teriparatide 120mg, risedronate sodium 30g, glucose 3000mg, appropriate amount of hydrochloric acid buffer.
[0031] The preparation process is as follows: accurately weigh the main drug in the prescribed amount, add appropriate amount of water for injection to dissolve; add the prescribed amount of mannitol and hydrochloric acid buffer to the above solution in turn, and adjust the pH to 6.5; Filter through a microporous membrane, set the volume to 2400ml, pack into 1000 tubes, press the plug, take out the box, tie the cover, and after passing the inspection, label and pack to obtain the composition injection.
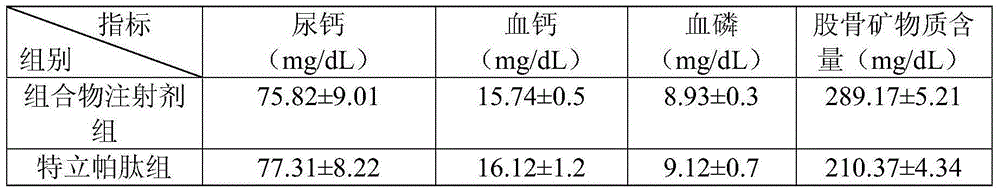
[0032] The therapeutic effect of the composition injection prepared in Example 2 on the osteoporosis rat model.
[0033] Experimental animals: 40 SD female rats, weighing about 200g, used 1% cellulose solution to add retinoic acid to adjust to an appropriate concentration, and administered 80mg / kg / d for 20 days, once a day, to form osteoporosis rats Model.
[...
Embodiment 3
[0039] Teriparatide 900mg, zoledronate sodium 4g, mannitol 3000mg, hydrochloric acid buffer in proper amount.
[0040] The preparation process is as follows: accurately weigh the main drug in the prescribed amount, add appropriate amount of water for injection to dissolve; add the prescribed amount of mannitol and hydrochloric acid buffer to the above solution in turn, and adjust the pH to 6.5; Filter through a microporous membrane, set the volume to 2400ml, pack into 1000 tubes, press the plug, take out the box, tie the cover, and after passing the inspection, label and pack to obtain the composition injection.
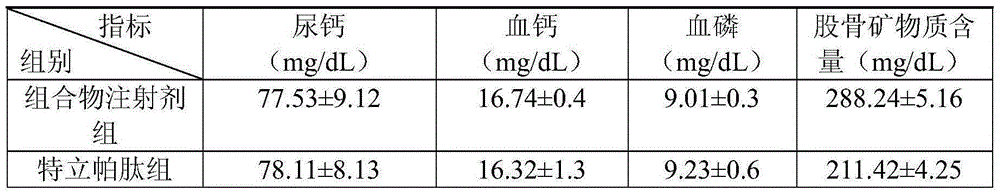
[0041] The therapeutic effect of the composition injection prepared in Example 3 on the osteoporosis rat model.
[0042]Experimental animals: 40 SD female rats, weighing about 200g, used 1% cellulose solution to add retinoic acid to adjust to an appropriate concentration, and administered 80mg / kg / d for 20 days, once a day, to form osteoporosis rats Model.
[0043] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com