Hyperphosphatemia treatment agent
A technology for hyperphosphatemia and therapeutic agent, applied in the field of hyperphosphatemia therapeutic agent, can solve problems such as decreased medication compliance, and achieve the effect of improving life prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
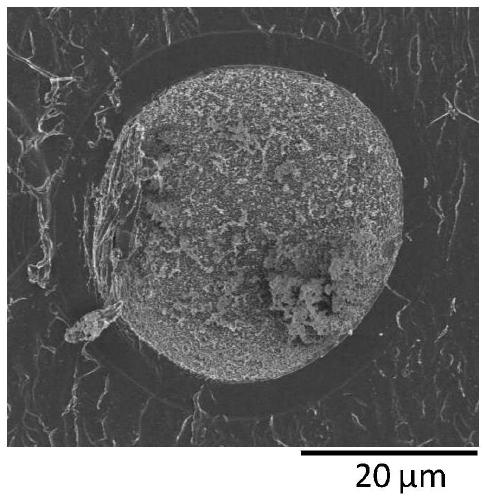
[0271] 400 g of a 15.0 mass % polyallylamine aqueous solution (PAA-15C manufactured by Nittobo Medical Co., Ltd., amine value: 17.5 mmol / g) was distilled off under reduced pressure to prepare 150 g of a 40.0 mass % polyallylamine aqueous solution (first solution ).
[0272] 318 g of a second solution was prepared by dissolving 15.0 g of ethyl cellulose (Ethyl cellulose (approximately 49% ethoxylated) 45 manufactured by Wako Pure Chemical Industries, Ltd., weight average molecular weight: 125,000) in 303 g of toluene.
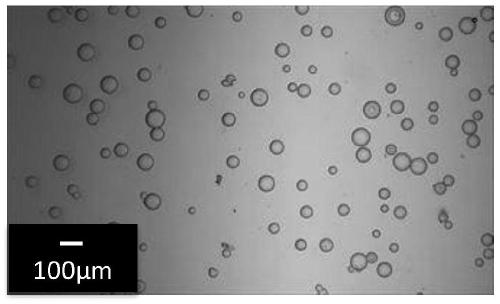
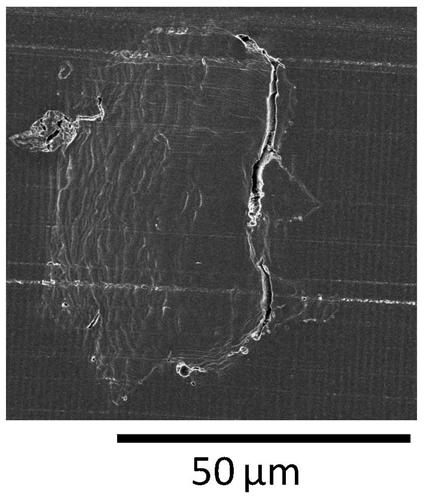
[0273] The above-mentioned first solution and the above-mentioned second solution were mixed in a 500 mL separable flask equipped with a Dean-Stark apparatus, thereby obtaining a mixture. Using stainless steel flat stirring blades (IKA R1375, blade diameter 70 mm) and Shinto Science Co., Ltd. Three-One Motor (BL600), the above mixture was stirred at 60° C. at 120 rpm for 60 minutes, by This gives a polyallylamine emulsion. Table 1 describes the physical proper...
Embodiment 2
[0286] 180 g of a 40.0 mass % polyallylamine aqueous solution (the first solution ).
[0287] 382 g of a second solution was prepared by dissolving 18.0 g of ethyl cellulose (Ethyl cellulose (approximately 49% ethoxylated) 45 manufactured by Wako Pure Chemical Industries, Ltd., weight average molecular weight: 125,000) in 364 g of toluene.
[0288] The above-mentioned first solution and the above-mentioned second solution were mixed in a 500 mL separable flask equipped with a Dean-Stark apparatus, thereby obtaining a mixture. Using stainless steel flat stirring blades (IKA R1375, blade diameter 70mm) and Shinto Science Co., Ltd. Three-One Motor (BL600), the above mixture was stirred at 50° C. at 120 rpm for 60 minutes. This gives a polyallylamine emulsion.
[0289] To the obtained emulsion, a solution obtained by diluting 4.90 g of 1,6-dichlorohexane (manufactured by Tokyo Chemical Industry Co., Ltd.) with 12 mL of toluene was added dropwise over 10 minutes. After completio...
Embodiment 3
[0291] Except having changed the temperature at the time of stirring from 50 degreeC to 80 degreeC, it carried out similarly to Example 2, and obtained the crosslinked polyallylamine spherical particle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com