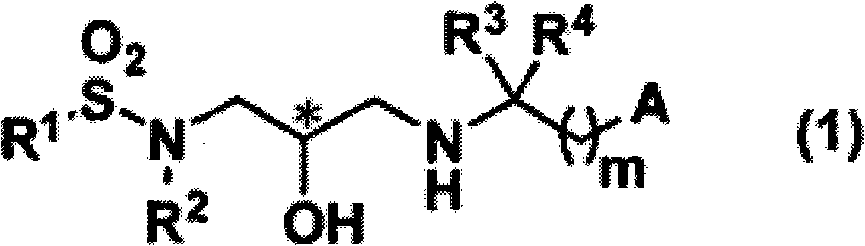
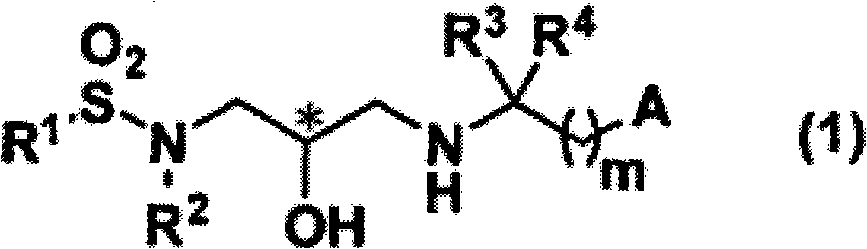
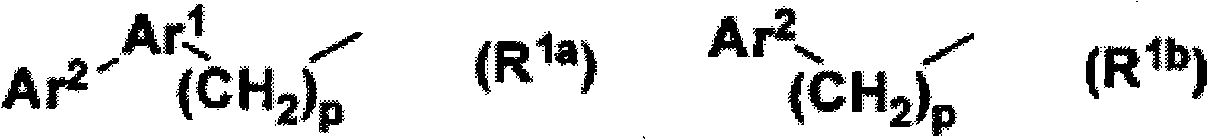Sulfonamide compound and application thereof
A compound and drug technology, applied in the field of novel sulfonamide compounds, can solve the problem of the absence of CaSR antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0318]Synthesis of (S)-2-bromo-N-methyl N-(oxiran-2-ylmethyl)benzenesulfonamide
[0319] (Step A) Synthesis of 2-bromo-N-methylbenzenesulfonamide
[0320] 2-Bromobenzenesulfonyl chloride (manufactured by Fluorochem, 25 g) was dissolved in tetrahydrofuran (40 mL), and a 40% aqueous methylamine solution (TCI, 25 mL) was added dropwise at 0°C over 10 minutes with stirring. After stirring at room temperature for 2 hours and 40 minutes, water was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. Hexane and a small amount of ethyl acetate were added to the residue, and after stirring, the solid was filtered to obtain the title compound (23.26 g).
[0321] (Step B) Synthesis of (S)-2-bromo-N-methyl N-(oxiran-2-ylmethyl)benzenesulfonamide
[0322] 2-Bromo-N-methylbenzenesulfonamide (10 g) synthesized by Example 1-...
Embodiment 1-2~6
[0324] According to the procedure A and procedure B of Example 1-1, instead of using 2-bromobenzenesulfonyl chloride, 3-bromobenzenesulfonyl chloride (Fluorochem), 4-bromobenzenesulfonyl chloride (TCI), (2-bromophenyl) Methanesulfonyl chloride (MAYB), (3-bromophenyl)methanesulfonyl chloride (MAYB) or (4-bromophenyl)methanesulfonyl chloride (MAYB) to give the title compound. The structures of the compounds of Examples 1-1 to 1-6 are shown below as Exp.1-1 to Exp.1-6, respectively.
[0325]
Embodiment 1-2
[0326] [Example 1-2] (S)-3-bromo-N-methyl-N-(oxirane-2-ylmethyl)benzenesulfonamide
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com