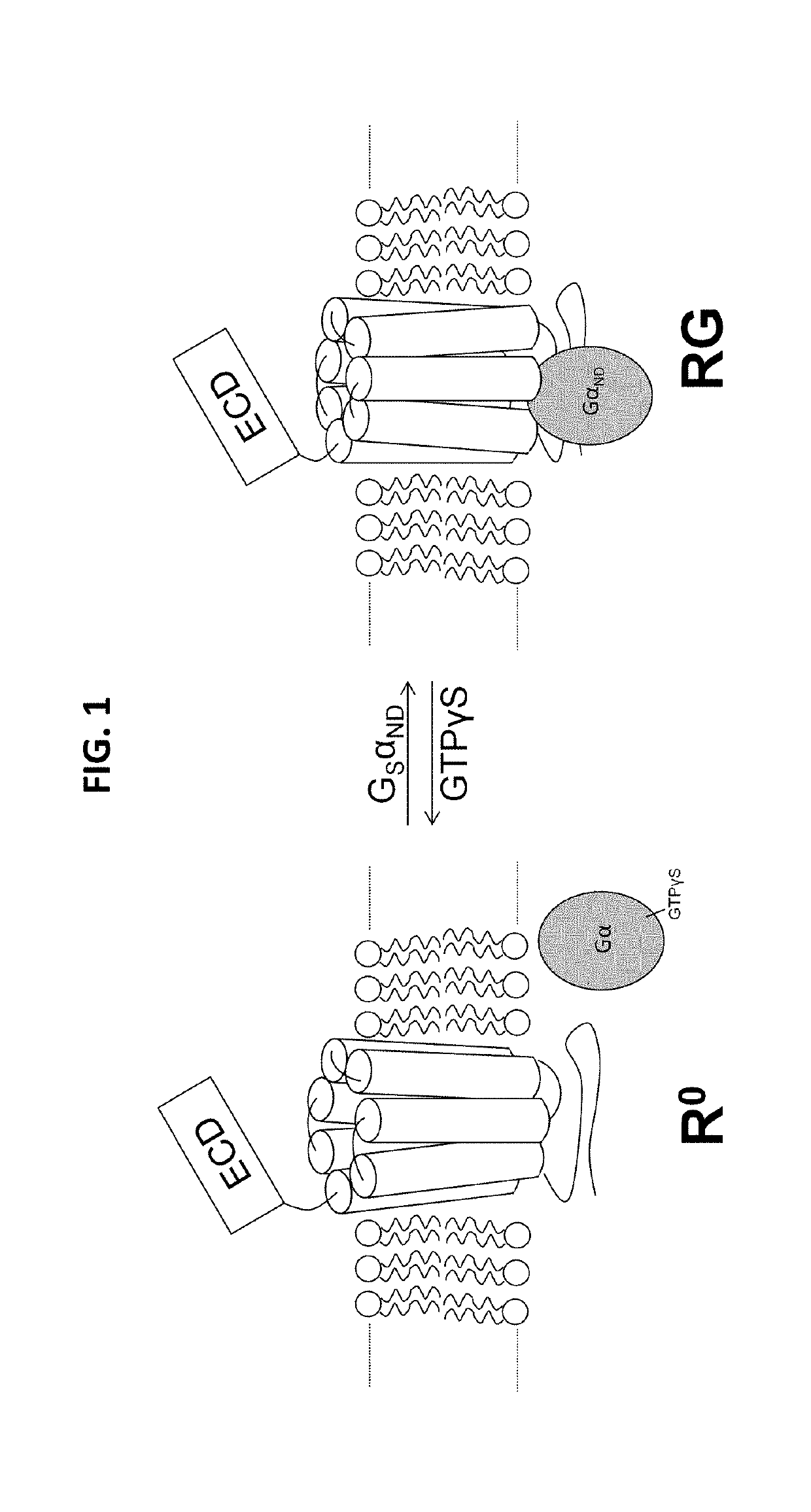
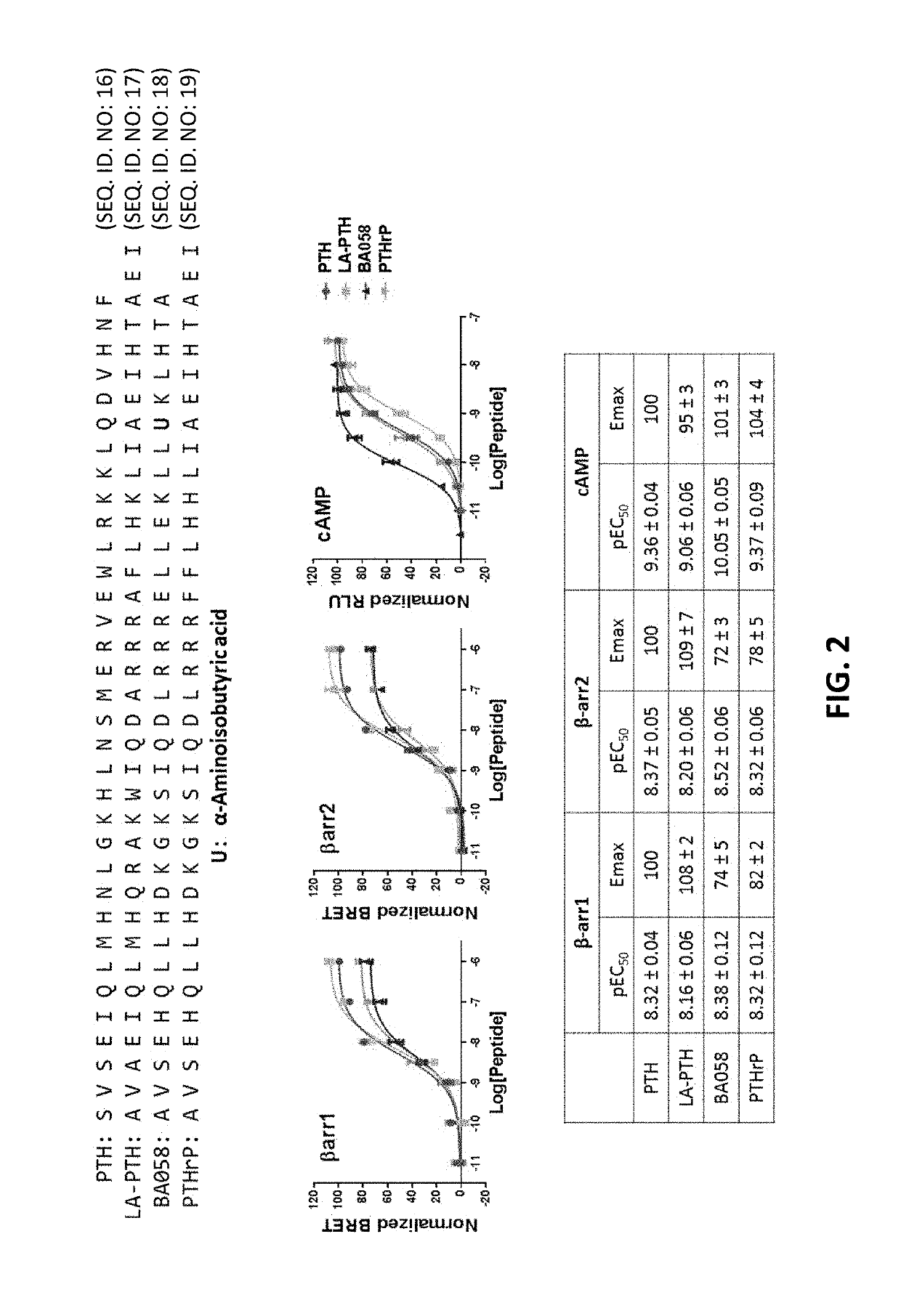
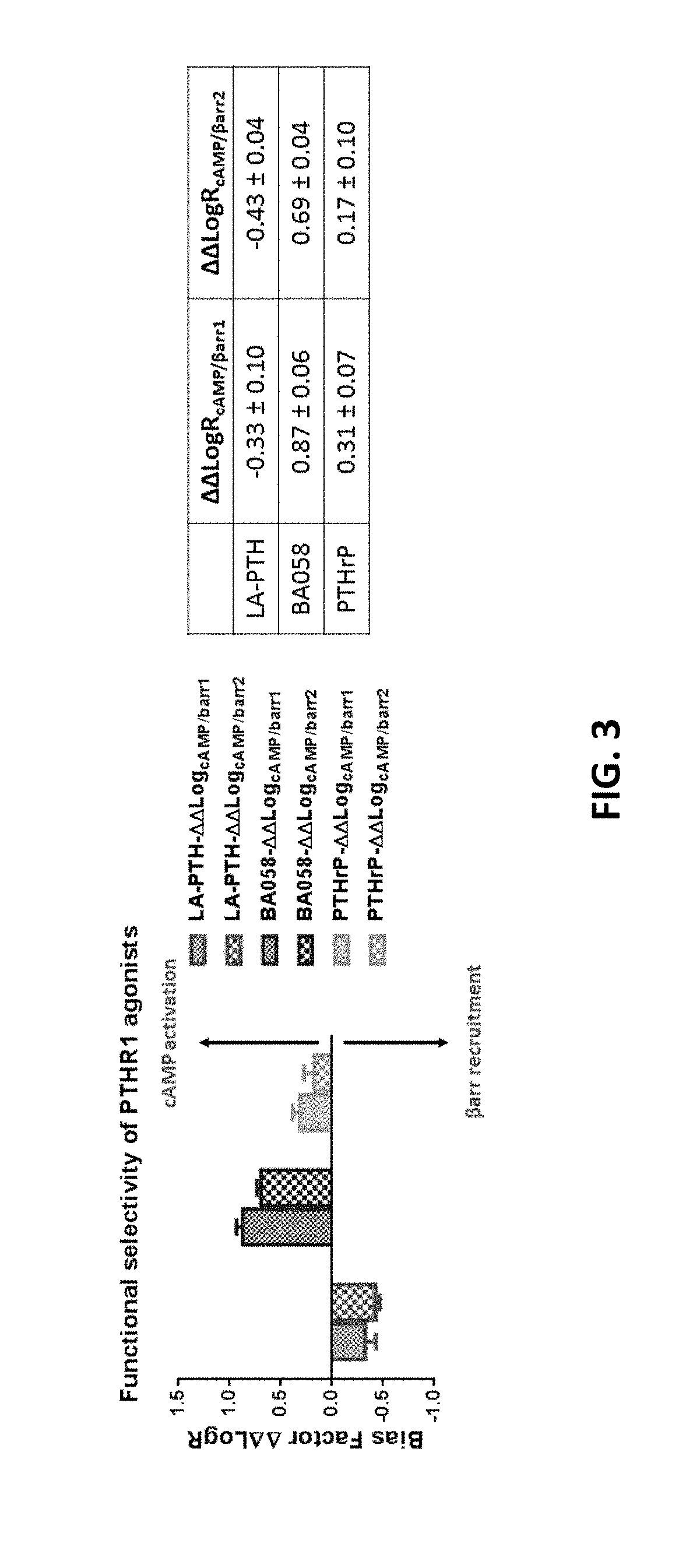
Analogues of parathyroid hormone (1-34) that function as agonists of the parathyroid hormone receptor-1 and display modified activity profiles
a technology of parathyroid hormone and receptor-1, which is applied in the field of analogues of parathyroid hormone, can solve the problems of low calcium in the blood, no rational method for designing such agonists, and people with hypoparathyroidism experience a number of unsettling symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Abbreviations and Definitions
[0027]Agonist, Antagonist, Inverse Agonist: An inverse agonist is an agent that binds to the same receptor as an agonist but induces a pharmacological response opposite to that agonist. A prerequisite for an inverse agonist response is that the receptor must have a constitutive level of activity in the absence of any ligand. An agonist increases the activity of a receptor above its basal level, whereas an inverse agonist decreases the activity below the basal level. An antagonist binds to the receptor and blocks the activity of both agonists and inverse agonists.
[0028]“Cyclically constrained” when referring to a β-amino acid or β-amino acid residue means a β-amino acid or β-amino acid residue in which the α-position and β-position carbon atoms in the backbone of the β-amino acid are incorporated into a substituted or unsubstituted C4 to C10 cycloalkyl, cycloalkenyl, or heterocycle moiety, wherein heterocycle moieties may have 1, 2, or 3 heteroatoms selec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com