Falecalcitriol controlled-release tablet
A technology of fluorocalcitriol and controlled-release tablets, which is applied in the field of medicine and can solve the problems of inconvenience in taking, low bioavailability, and reducing the number of times of taking medicines.
Inactive Publication Date: 2020-10-27
CP PHARMA QINGDAO CO LTD
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0006] In order to solve the disadvantages of inconvenient taking and low bioavailability of the existing fluorocalciotriol preparations, the present invention invented fluorocalciotriol controlled-release tablets, which can reduce the number of times of taking medicine, slow down the absorption rate, prolong the biological half-life, and control the blood drug concentration at Within the effective blood concentration range, thereby reducing side effects and improving patient compliance
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
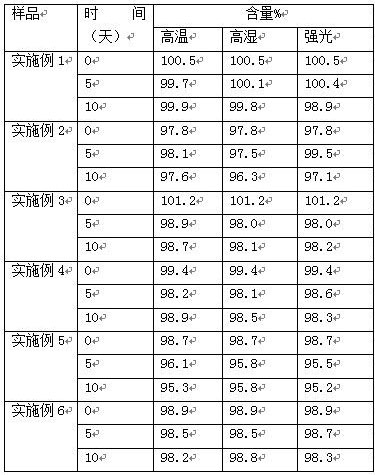
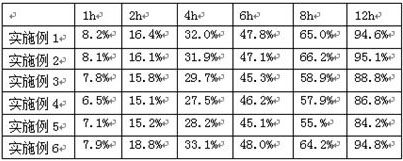
Effect test
Embodiment 1-6
[0020] Preparation of Example 1-6 Fluoceotriol Controlled-release Tablets
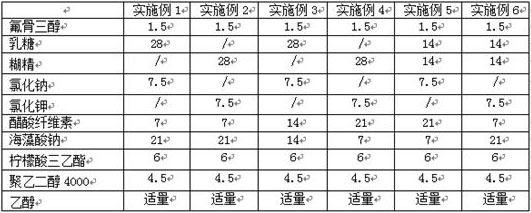
[0021] According to the raw and auxiliary materials in the table below, according to the above-mentioned preparation method, six examples of fluocaustrol controlled-release tablets were prepared, wherein " / " means not used.
[0022] Table 1 Examples 1-6
[0023]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a preparation method of a falecalcitriol controlled-release tablet, and belongs to the technical field of medicines. The falecalcitriol controlled-release preparation in the invention is composed of a tablet containing core and a coating film wrapping the tablet containing core; the tablet containing core comprises falecalcitriol, a filler and sodium chloride; the coating film comprises a controlled-release material, a plasticizer and a pore-foaming agent; when preparation, the tablet containing core is wrapped by the coating film, wherein the main component, namely falecalcitriol, belongs to the vitamin D3 analogue, can adjust calcium metabolism and resist rickets, and can be used for treating hypoparathyroidism. The drug in the invention can be released slowly anduniformly; the purposes of being long to act and increasing the curative effect are achieved; the dosage also can be reduced when the same drug effect is kept; therefore, side effects of taking drugsto patients are reduced; furthermore, the preparation process is simple; and the obtained product is steady in quality and suitable for large-scale production and application.
Description
technical field [0001] The invention belongs to the technical field of medicine, and relates to a preparation method of flucitriol controlled-release tablets. The invention provides a safe, effective, stable quality, low cost, less frequency of administration, enhanced patient compliance, and stable pain relief. Controlled-release preparations for spasms. Background technique [0002] Calcitriol is an analogue of vitamin D3 and a derivative of the active form of the small intestine. It plays a role by binding to receptors distributed in target tissues. This product can regulate calcium metabolism and resist rickets. It is used for the treatment of parathyroid function Hypoplasia. Existing calcitriol preparations are mainly tablets, and calcitriol tablets were jointly developed and marketed by Sumitomo and Taisho Pharmaceutical in Japan at the earliest in April 2001; they are not listed in other countries. [0003] The parathyroid glands are endocrine glands mostly located ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/36A61K31/593A61K47/38A61K47/36A61P19/08A61P3/14A61P5/14
CPCA61K9/286A61K9/2866A61K31/593A61P3/14A61P5/14A61P19/08
Inventor 孙桂玉刘晓霞陈阳生臧云龙王明刚杜昌余牛建兴薛文静王婷婷
Owner CP PHARMA QINGDAO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com