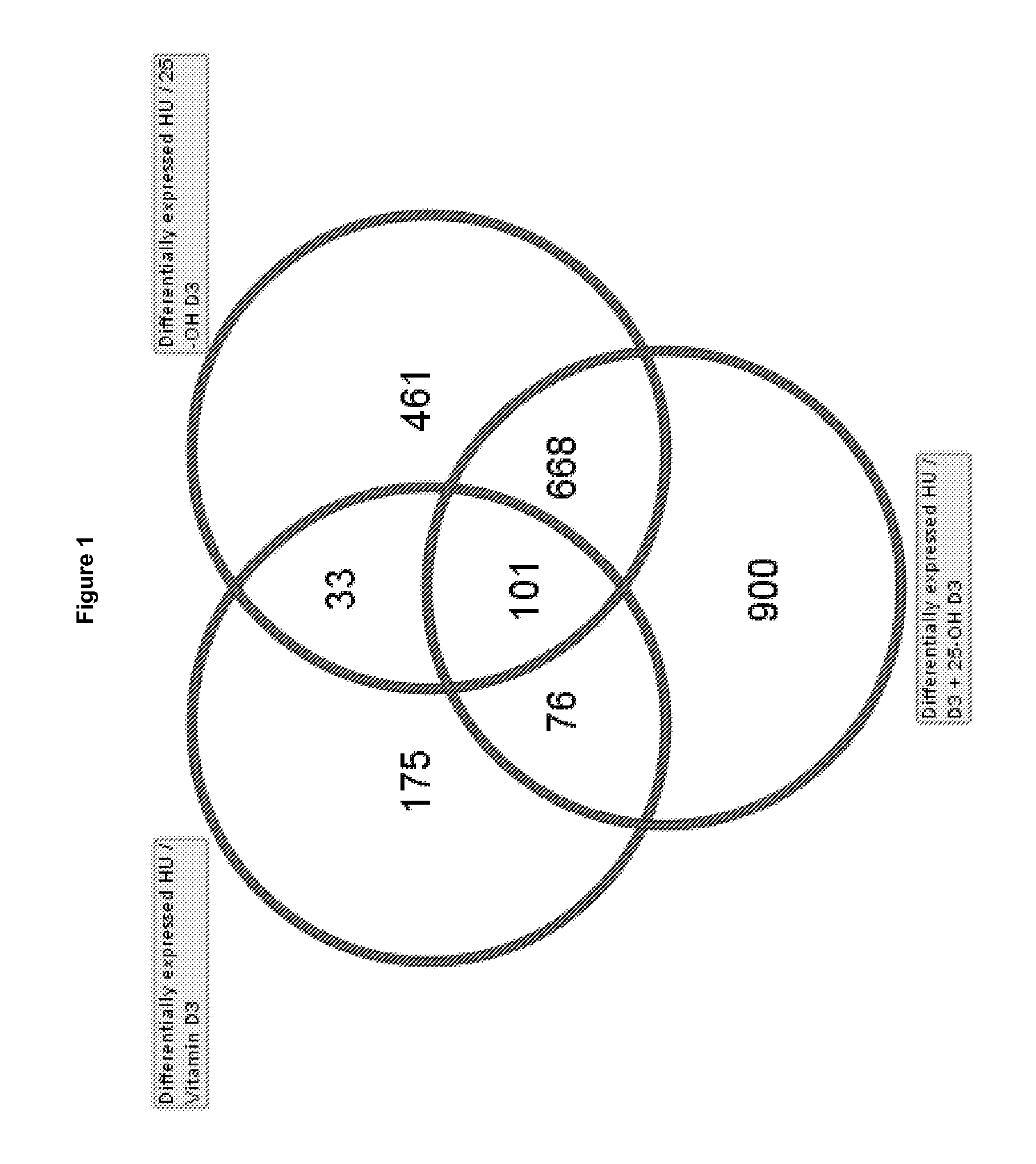
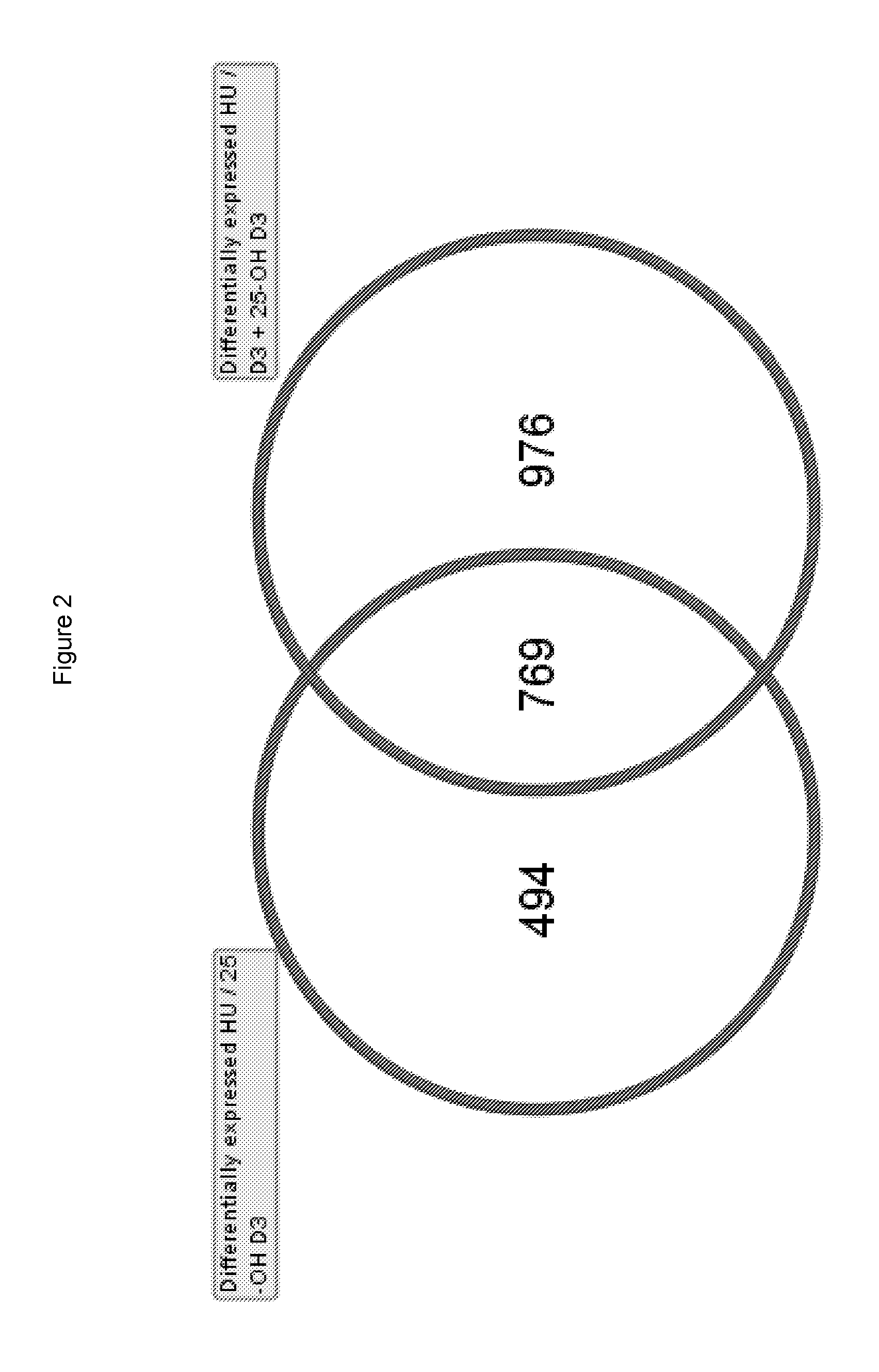
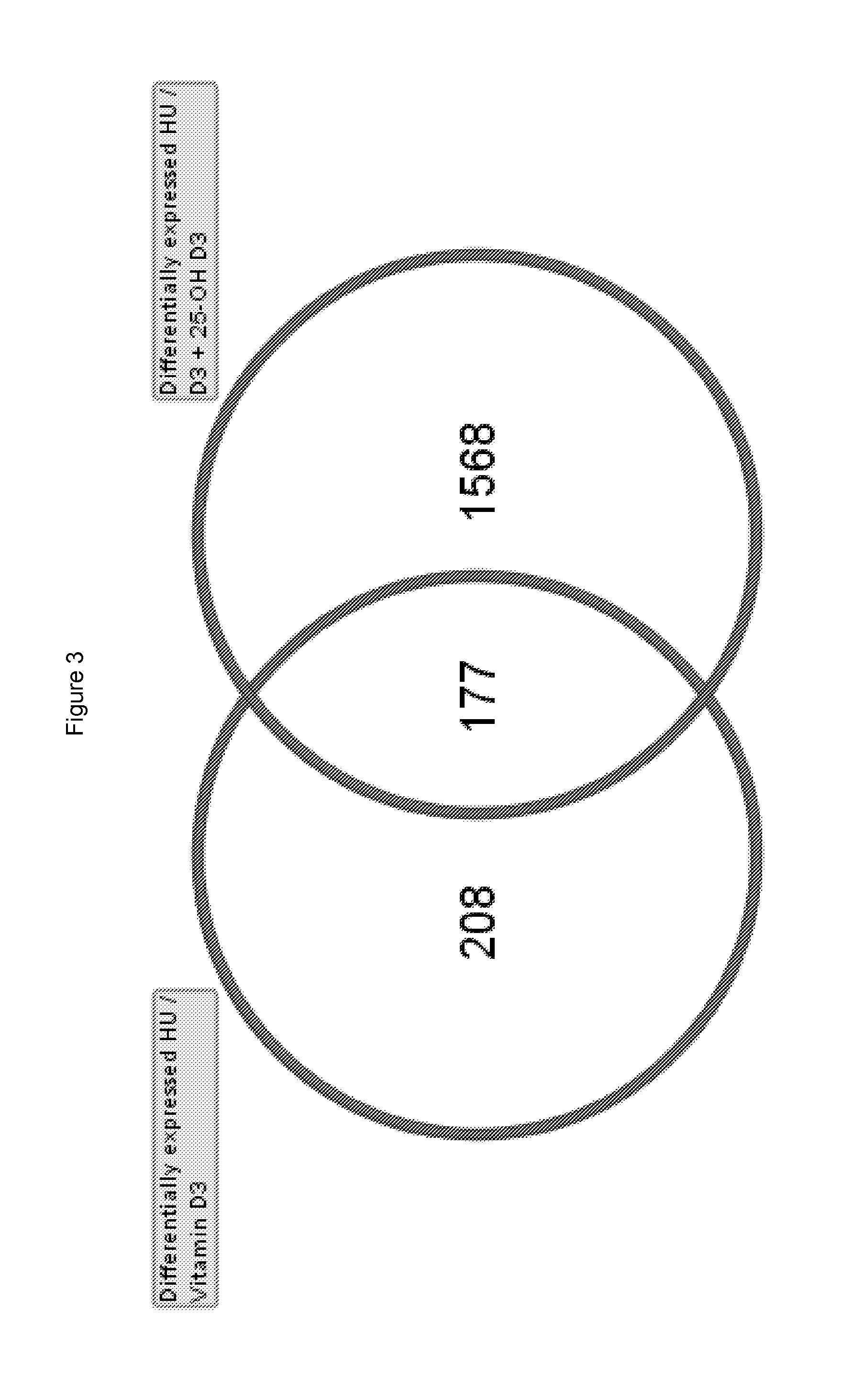
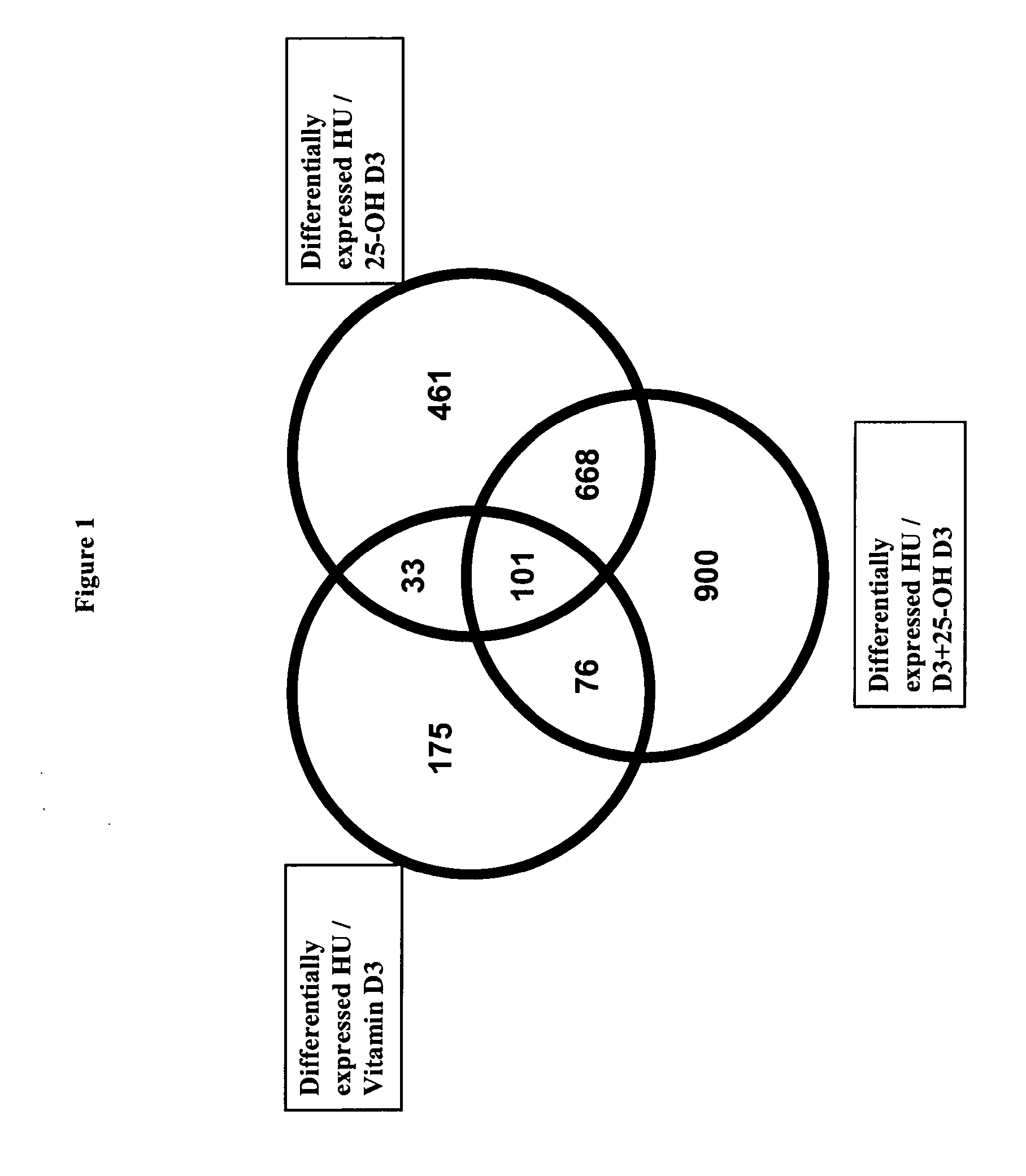
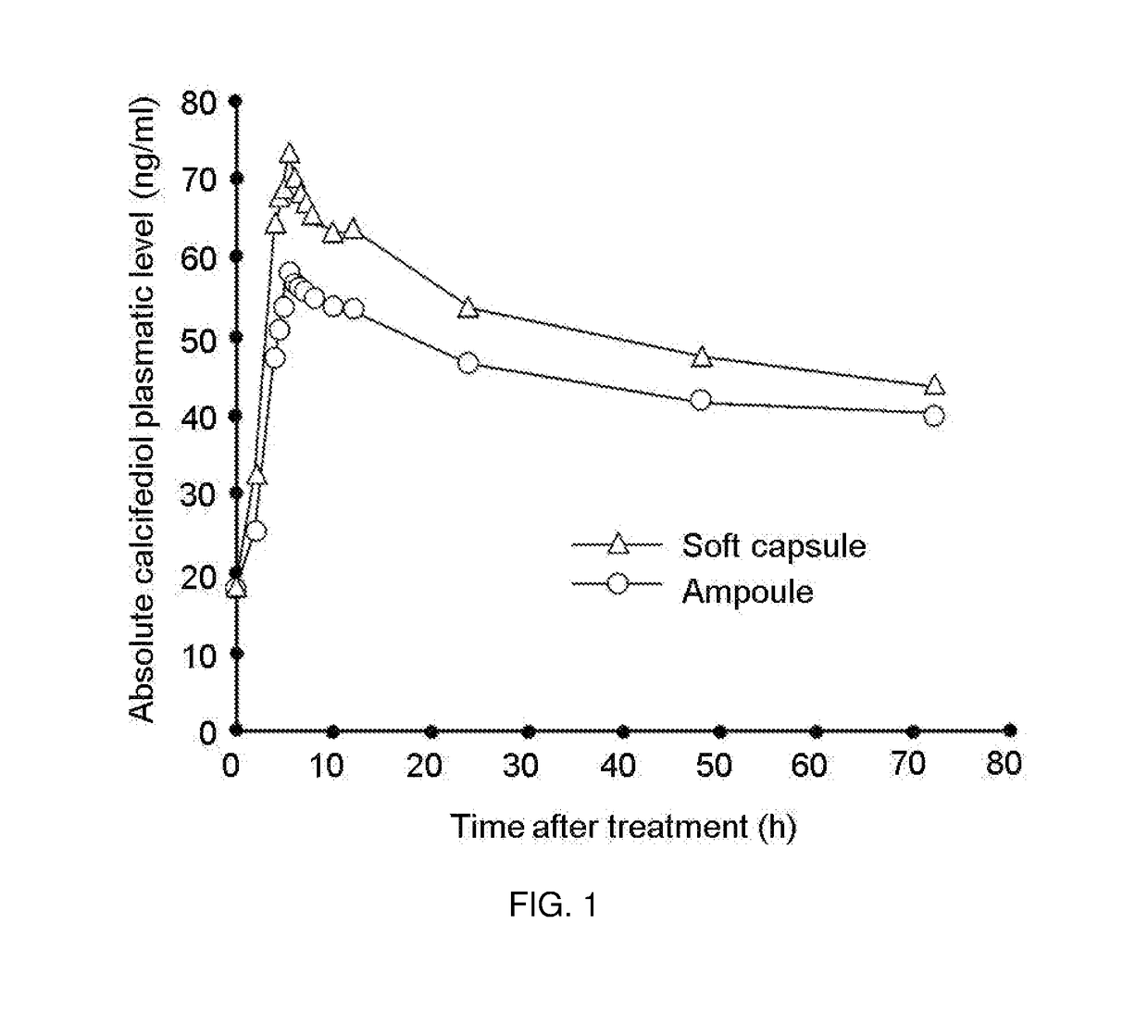
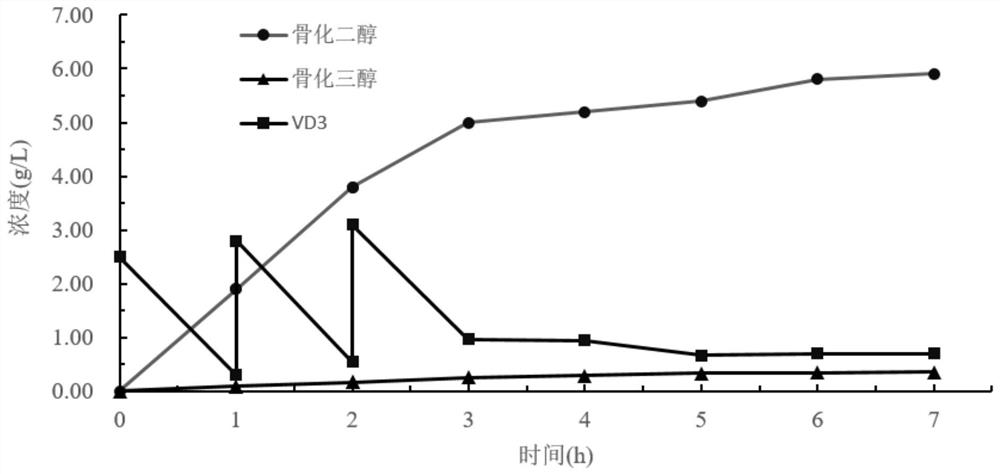
Patents
Literature
39 results about "Calcifediol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat people with serious kidney disease whose bodies make too much of a certain natural substance (parathyroid hormone-PTH).
Use of 25-hydroxy-vitamin d3 to affect human muscle physiology
InactiveUS20110039810A1Function increaseIncrease muscle strengthOrganic active ingredientsBiocideMuscle strengthPhysiology
Owner:DSM IP ASSETS BV
Treating hyperglycemia with 25-hydroxyvitamin d3
InactiveUS20110039811A1Reduce high blood glucoseMaintain blood glucoseBiocideOrganic active ingredientsCalciferolsPharmaceutical drug
We disclose treating hyperglycemia in a human with 25-hydroxyvitamin D3 (calcifediol). Blood glucose is reduced to a level which is closer to normal than baseline. Vitamin D3 (cholecalciferol) may optionally be used together with 25-hydroxy vitamin D3. Forms and dosages of a pharmaceutical composition, as well as processes for manufacturing medicaments, are also disclosed.
Owner:DSM IP ASSETS BV
Combination of vitamin d and 25-hydroxyvitamin d 3
InactiveUS20110052707A1Quick correctionSufficient supplyBiocideOrganic chemistryErgocalciferolMedicine
We disclose compositions comprising Vitamin D (cholecalciferol and / or ergocalciferol) and 25-OH D3 (calcifediol), and use of those compositions to affect at least concentration, bioavailability, metabolism, or efficacy of vitamin D in a human. Forms and dosages of the composition, as well as processes for manufacturing a spray-dried formulation, are also disclosed.
Owner:DSM IP ASSETS BV
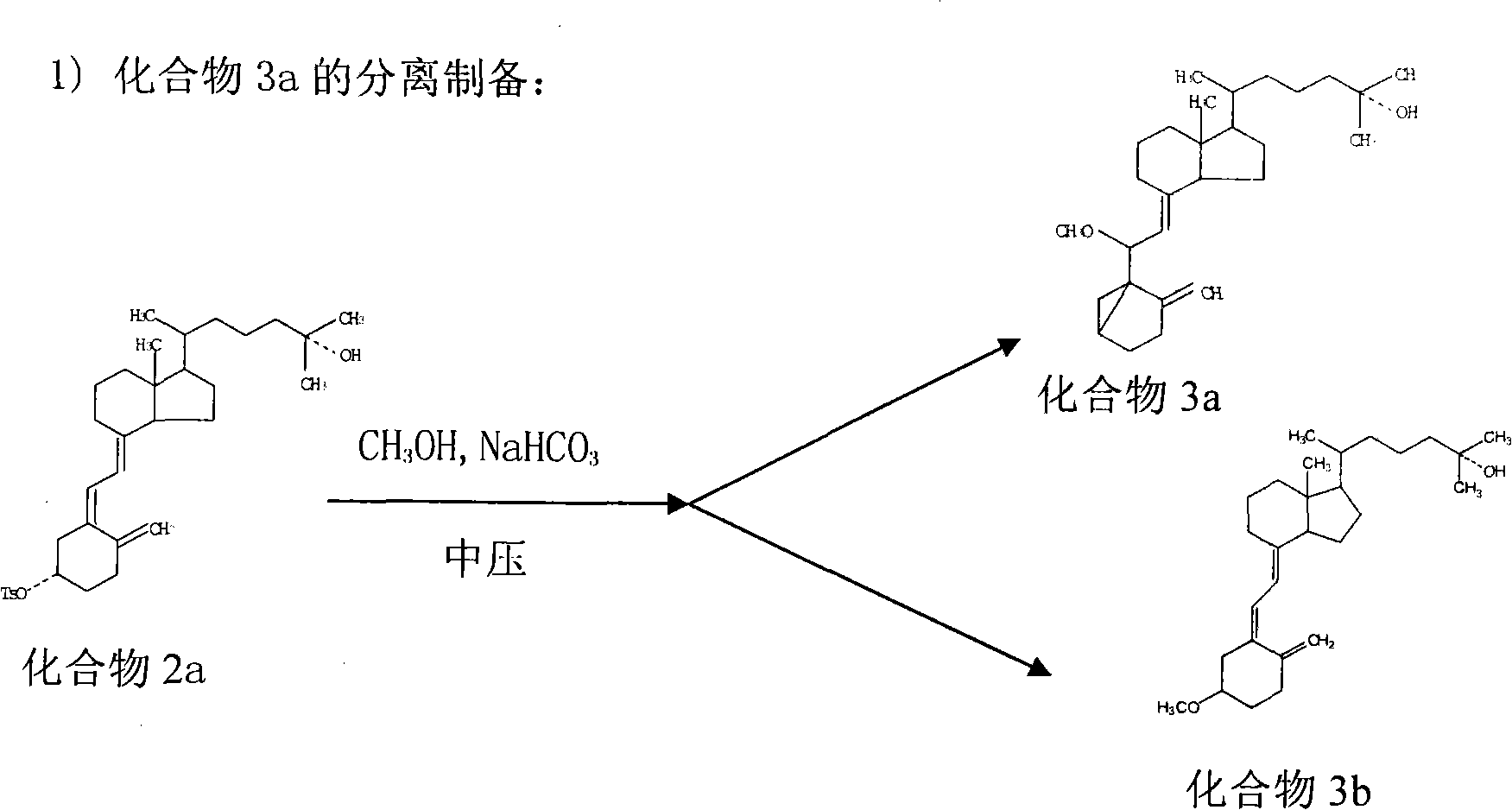
Preparation method of calcitriol
ActiveCN101607931AEfficient removalEfficient separationIon-exchange process apparatusOrganic chemistryChromatographic separationChemical reaction
The invention relates to a preparation method of calcitriol which is a compound material, belonging to the technical field of preparation of drug synthesis and organic compound synthesis. In the invention, the calcifediol is taken as raw material, a majority of produced impurities in the chemical reaction process are removed through medium-pressure liquid phase chromatography, wherein calcifediol carried out sulfonylation and ring closing on toluene, and the medium-pressure liquid phase chromatography is used for removing the impurities; products carry out oxidation reaction, medium-pressure chromatographic column separates and removes the impurities, ring opening is carried out, medium-pressure liquid phase is added for coarsely separating stereoisomeride, and at last the products separated by high-pressure liquid phase chromatography is hydrolyzed and refined to obtain the calcitriol. The invention is reasonably selected, comprehensively applies the advantages of the medium and high pressure liquid phase chromatography, has simple detaching method, can effectively remove the impurities, thoroughly separates the stereoisomeride, has high purity of the calcitriol obtained from separation, has a productive rate of 40 percent, high yield and low cost, shortens production period, is suitable for industrial scale, has application prospect, and provides a time-saving, economic and efficient method for the separation and the preparation of chiral compounds.
Owner:CP PHARMA QINGDAO CO LTD
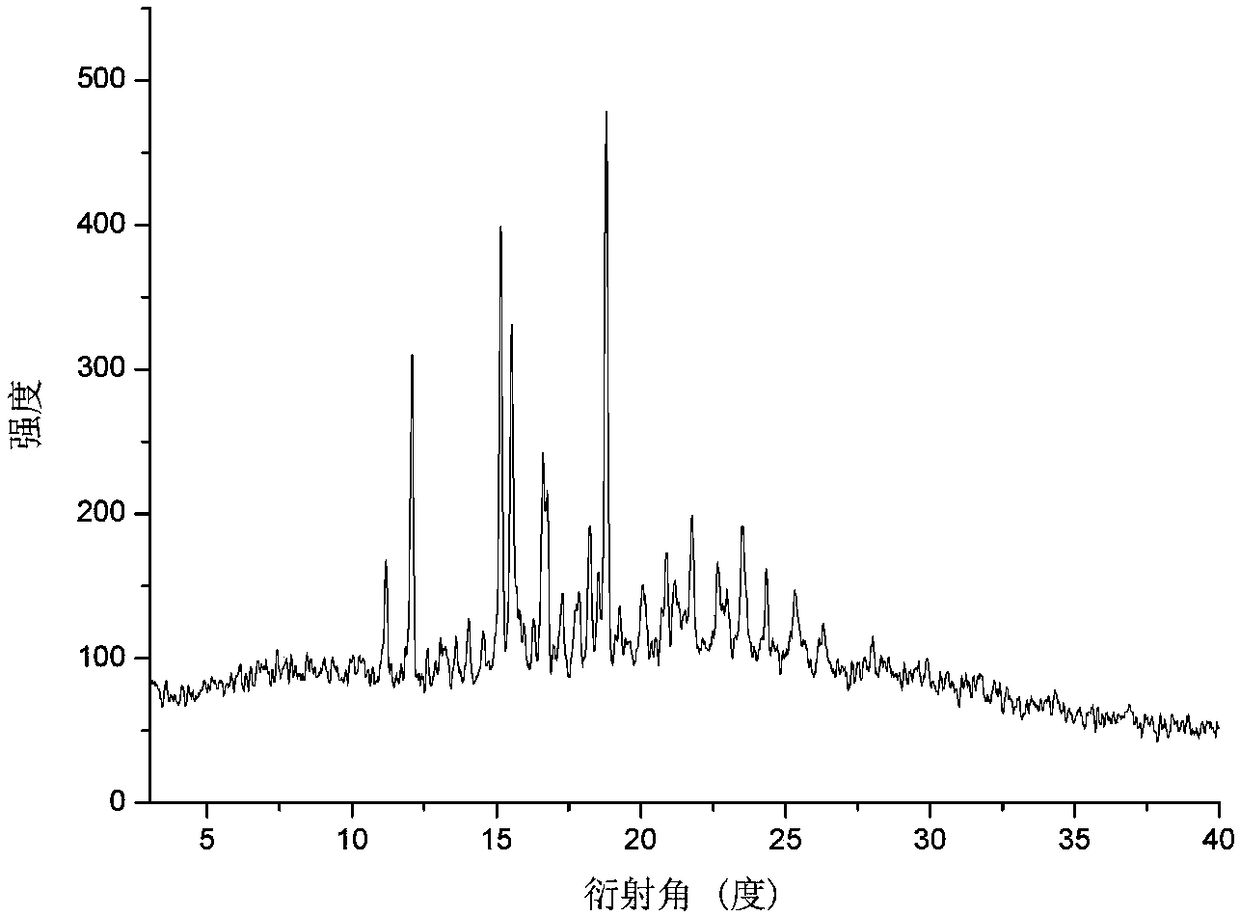
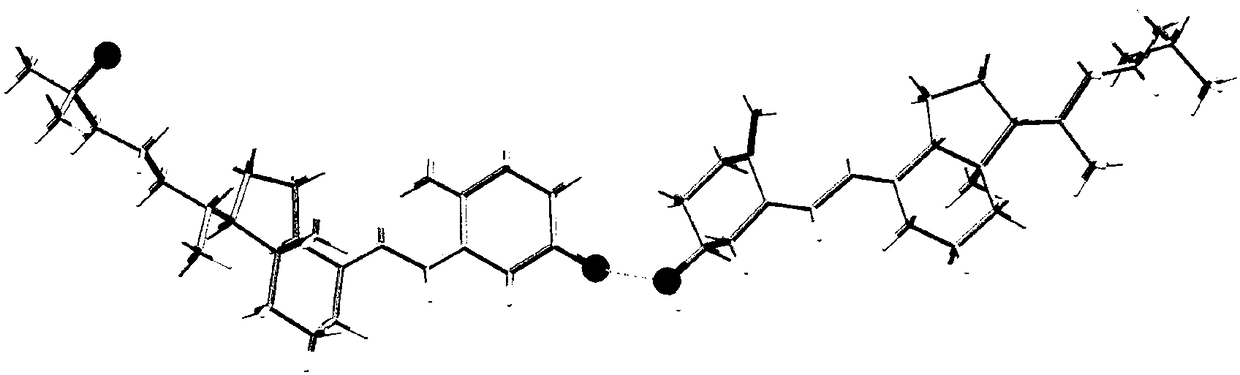
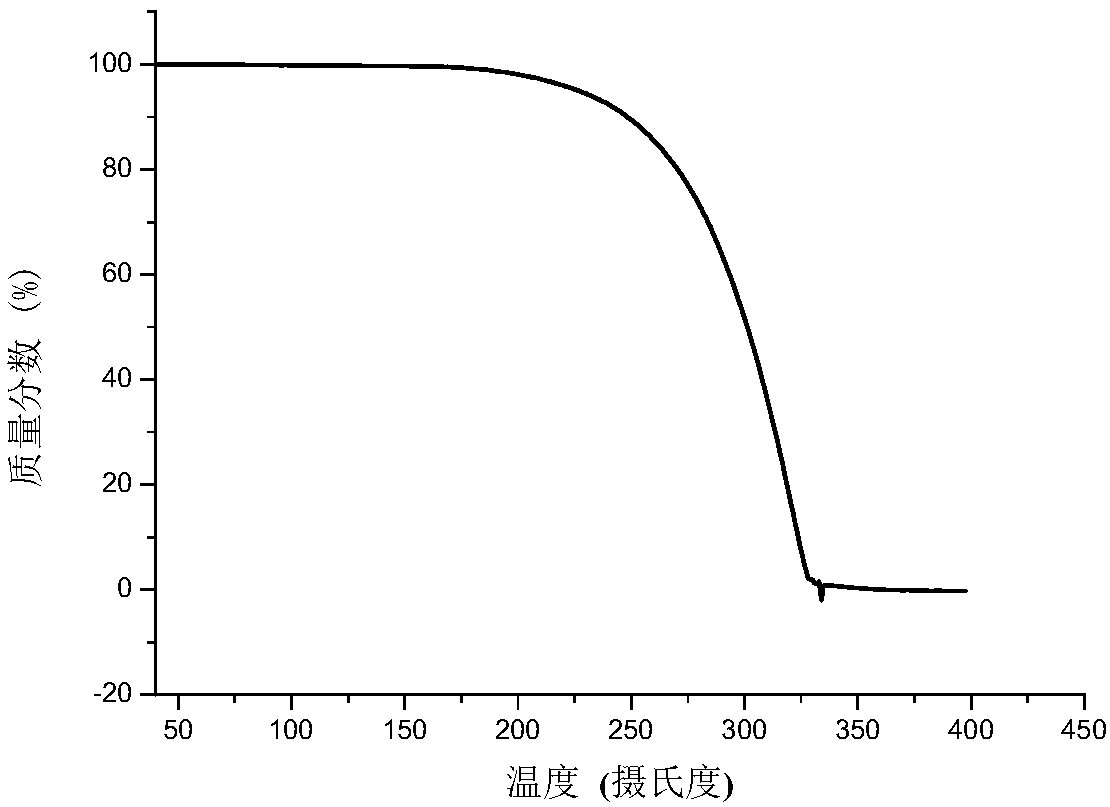
Eutectic crystal of calcifediol and vitamin D3 as well as preparation method and application thereof
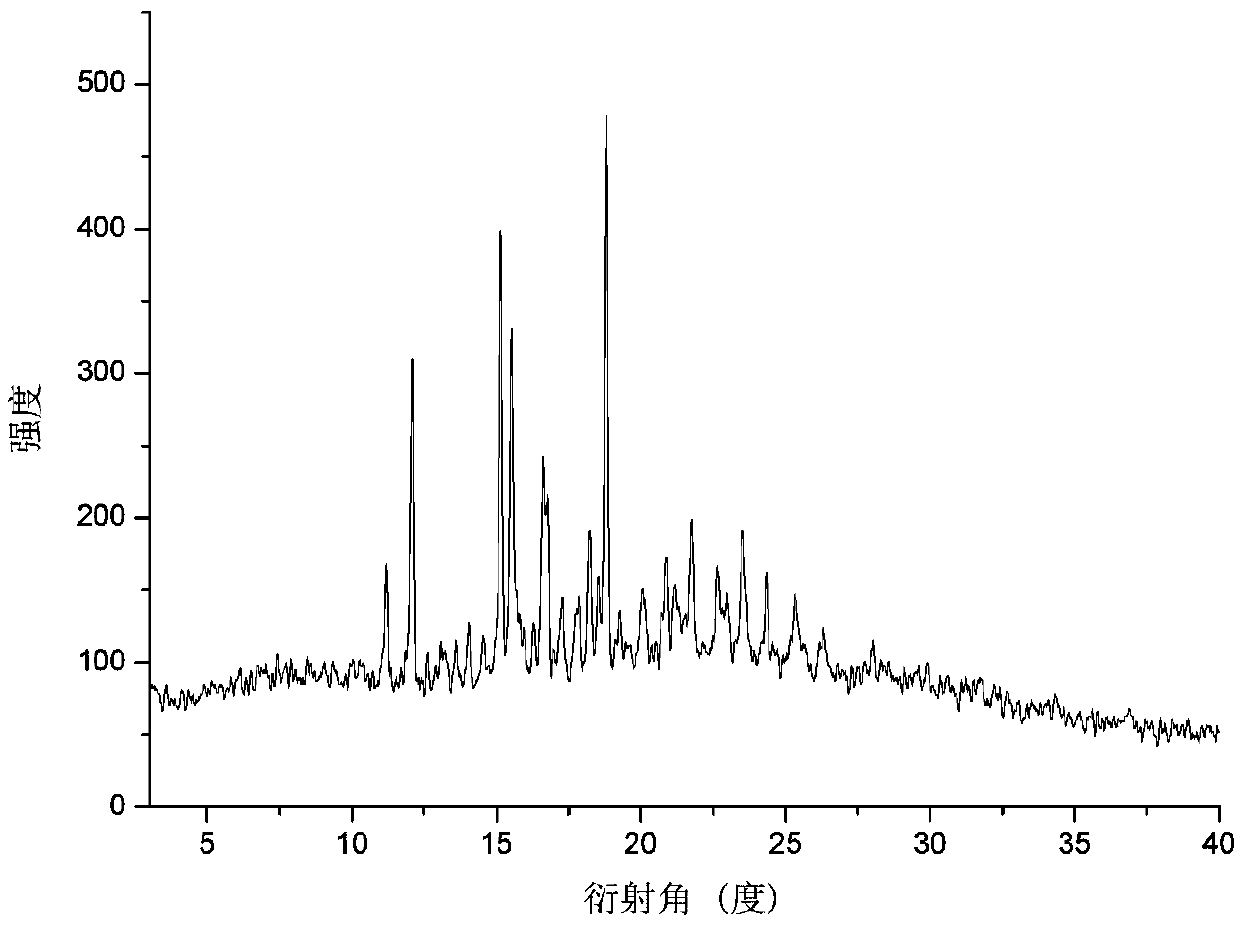
InactiveCN108129371AGood chemical stabilityImprove complianceOrganic active ingredientsMetabolism disorderSingle Crystal DiffractionX-ray
The invention relates to a eutectic crystal of calcifediol and vitamin D3 as well as a preparation method and application thereof. In the eutectic crystal, the mole ratio of the calcifediol to the vitamin D3 is 1 to 1. The eutectic crystal of the calcifediol and the vitamin D3 is subjected to comprehensive characterization by applying measures such as X-ray single crystal diffraction, X-ray powderdiffraction, thermogravimetric analysis, differential scanning calorimetry and infrared spectroscopic analysis, thereby finding that the chemical stability of the vitamin D3 of the eutectic crystal can be obviously improved under an illumination condition. The preparation method of the eutectic crystal of the calcifediol and the vitamin D3 is simple, and the eutectic crystal can be repeatedly prepared.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Combination of vitamin d and 25-hydroxyvitamin d 3
InactiveCN106214683ARapid correction of plasma concentrationsSufficient supplyOrganic active ingredientsMetabolism disorderErgocalciferolSterol
We disclose compositions comprising Vitamin D (cholecalciferol and / or ergocalciferol) and 25-OH D3 (calcifediol), and use of those compositions to affect at least concentration, bioavailability, metabolism, or efficacy of vitamin D in a human. Forms and dosages of the composition, as well as processes for manufacturing a spray-dried formulation, are also disclosed.
Owner:DSM IP ASSETS BV
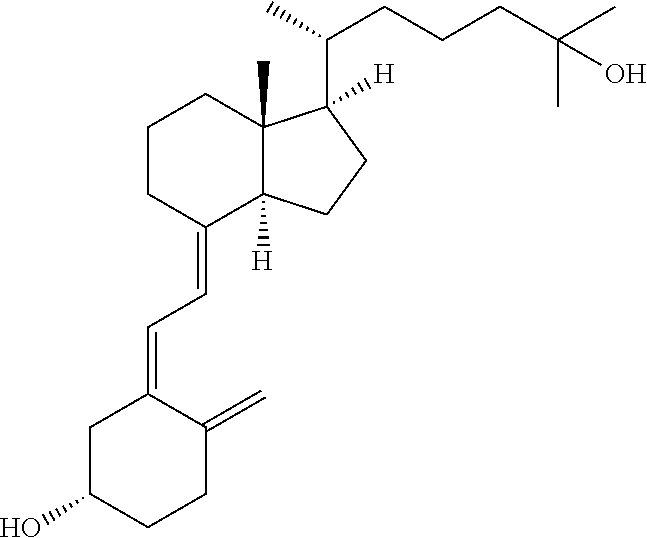
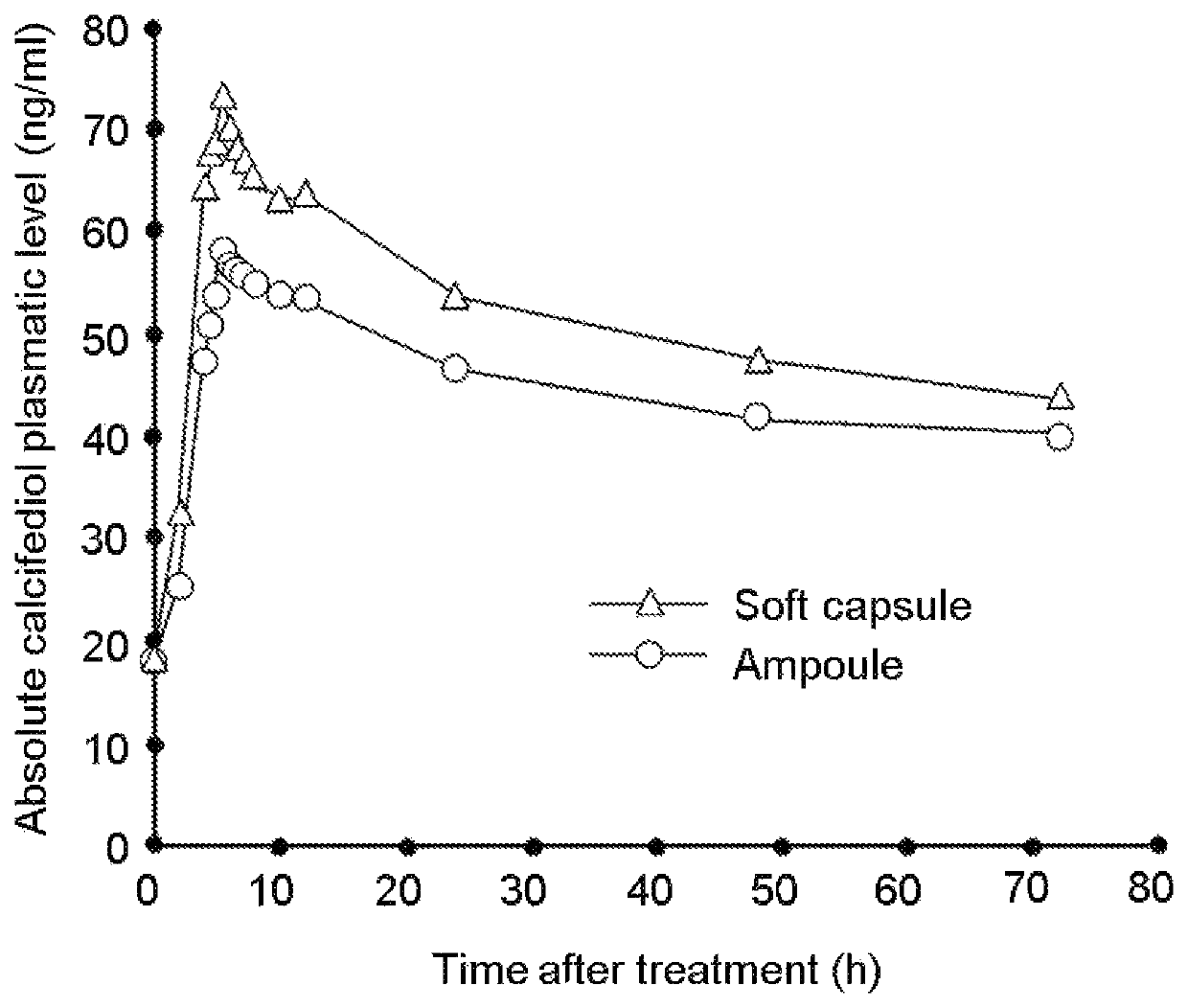
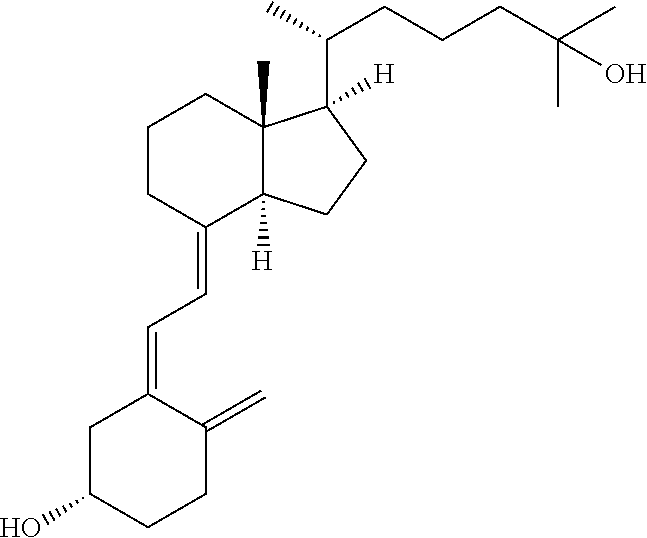
Calcifediol soft capsules
ActiveUS20170348249A1Improve bioavailabilityHydroxy compound active ingredientsAntipyreticRenal osteodystrophyHypophosphatemia
The present invention relates to calcifediol soft capsules, to their use in the treatment or prevention of diseases related to vitamin D deficiency, such as vitamin D deficiency, demineralization such as hypocalcemia and hypophosphatemia, renal osteodystrophy, rickets, osteoporosis, osteopenia, osteoarthritis, osteoarthrosis, osteomalacia, hypoparathyroidism, and inflammatory bowel disease, and to their process of manufacture.
Owner:FAES FARMA SA
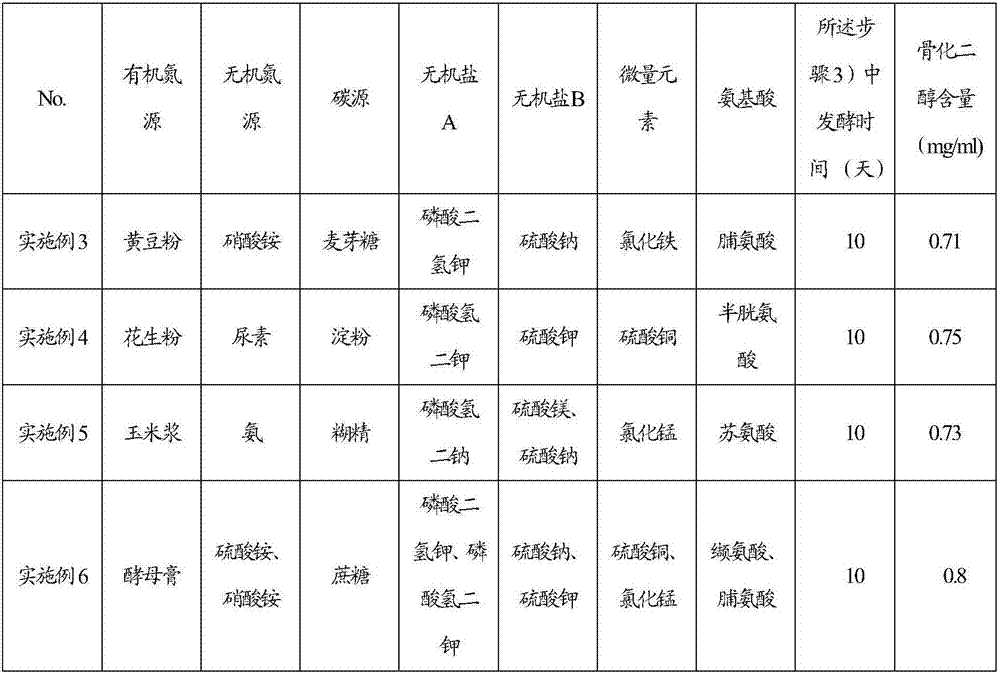
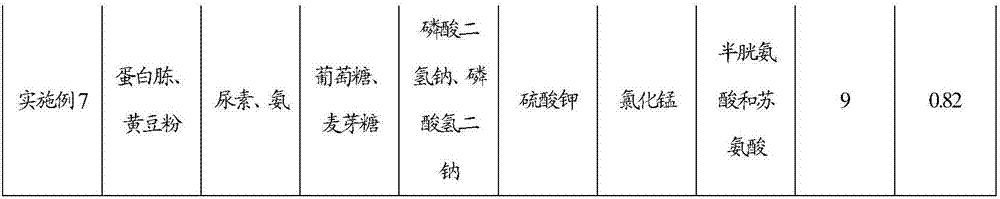
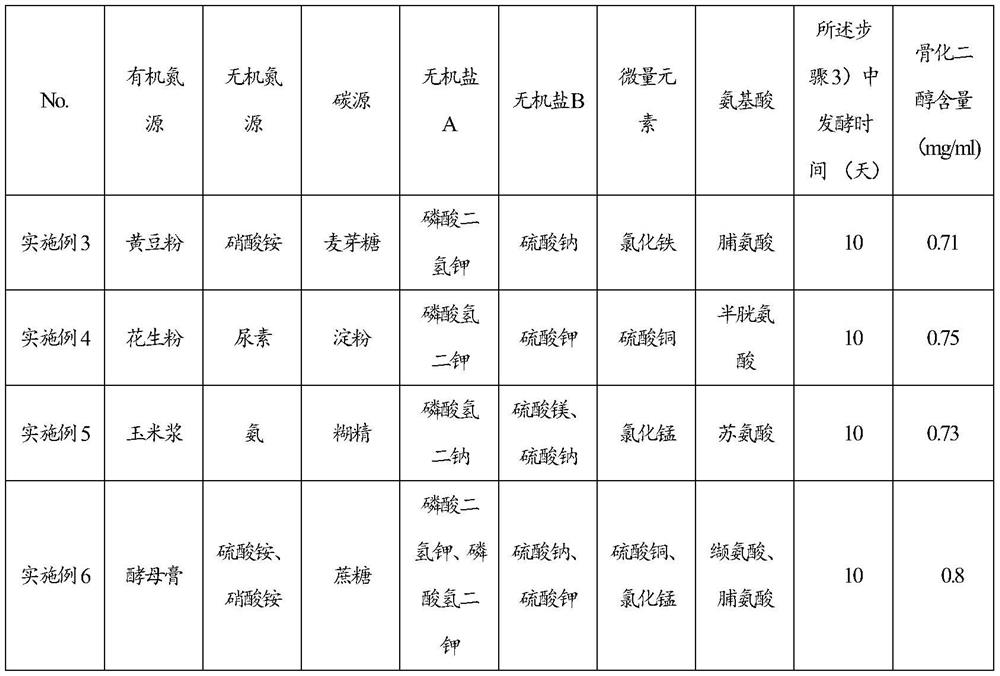
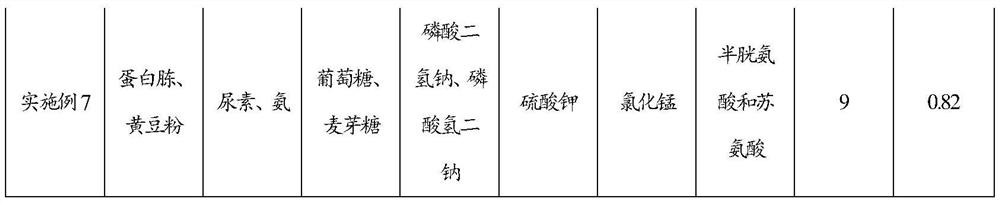
Feeding culture medium by fermenting calcifediol, feeding method for fermentation and fermentation method of calcifediol
ActiveCN107365805ASatisfy the dissolved oxygen demand of fermentationAvoid Fermentation Oxygen RequirementsBacteriaMicroorganism based processesInorganic saltsDissolution
The invention discloses a feeding culture medium by fermenting calcifediol, a feeding method for fermentation and a fermentation method of the calcifediol. The feeding culture medium comprises, by concentration, 0.2-3.0g / 100ml of an organic nitrogen source, 0.2-3.0g / 100ml of an inorganic nitrogen source, 0.5-3.0g / 100ml of a carbon source, 0.1-1.0g / 100ml of inorganic salt A, 0.1-1.0g / 100ml of inorganic salt B and 0.0002-0.0006g / 100ml of a microelement. The feeding method and the fermentation method comprise the steps that the feeding culture medium and water are added into fermentation broth, therefore the requirements for nutrient ingredients of fermentation of the calcifediol are satisfied, the requirements for oxygen dissolution of fermentation of the calcifediol are satisfied, the power cost is lowered, and the fermentation efficiency is improved.
Owner:太原市威尔潞威科技发展有限公司 +1
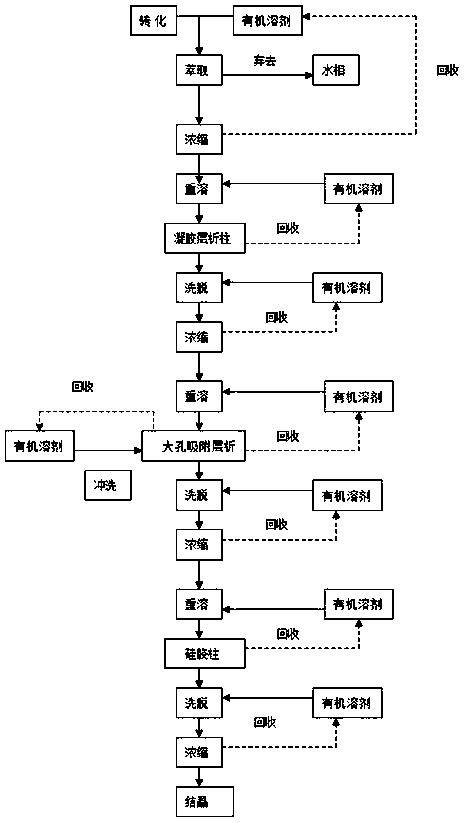
A novel calcifediol (25-hydroxyvitamin D3) separation and purification method
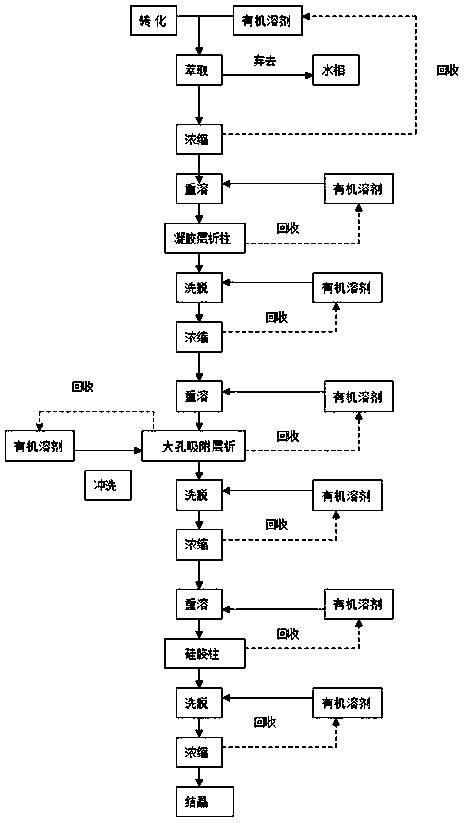
A novel calcifediol (25-hydroxyvitamin D3) separation and purification method is provided. The method mainly includes subjecting calcifediol to extraction and separation with an organic solvent; concentrating an extract liquid; dissolving the concentrate again to obtain a calcifediol stock solution for column chromatography; preliminarily purifying the calcifediol product by utilizing a gel chromatographic column; then further purifying and separating the calcifediol product by utilizing macroporous adsorption resin; finally preparing a high-purity calcifediol product through column chromatography on silica gel; subjecting an eluate to concentration and crystallization to obtain a calcifediol crystal product. The method is advantageous in that 1) the calcifediol product obtained by separation and purification through the method is high in purity and high in yield; 2) gel column chromatography and macroporous resin adsorption chromatography are applied for separation and purification ofthe calcifediol product for the first time; 3) the method is simple in process and convenient to operate, a plurality of times of cyclic operation can be performed, the equipment cost is low and themethod is particularly suitable for industrial promotion; 4) organic solvents adopted in the method can be recycled and reused, so that the production cost is reduced, and the method is economical andenvironmentally friendly.
Owner:山东惠仕莱生物科技有限公司
Combination of vitamin d and 25-hydroxyvitamin d 3
InactiveUS20160324877A1Pronounced and long plateau of 5-OHQuick correctionOrganic active ingredientsMetabolism disorderErgocalciferolMedicine
We disclose compositions comprising Vitamin D (cholecalciferol and / or ergocalciferol) and 25-OH D3 (calcifediol), and use of those compositions to affect at least concentration, bioavailability, metabolism, or efficacy of vitamin D in a human. Forms and dosages of the composition, as well as processes for manufacturing a spray-dried formulation, are also disclosed.
Owner:DSM IP ASSETS BV
Calcifediol soft capsules
ActiveUS10525018B2Improve bioavailabilityHydroxy compound active ingredientsAntipyreticJoint diseaseOsteo arthritis
The present invention relates to calcifediol soft capsules, to their use in the treatment or prevention of diseases related to vitamin D deficiency, such as vitamin D deficiency, demineralization such as hypocalcemia and hypophosphatemia, renal osteodystrophy, rickets, osteoporosis, osteopenia, osteoarthritis, osteoarthrosis, osteomalacia, hypoparathyroidism, and inflammatory bowel disease, and to their process of manufacture.
Owner:FAES FARMA SA
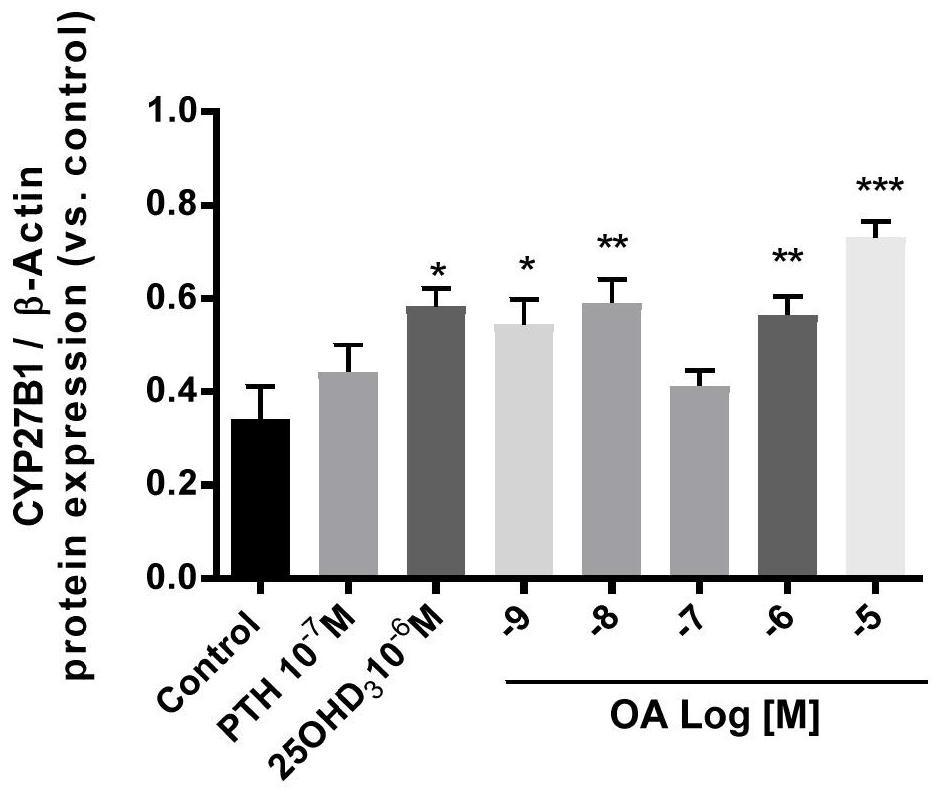
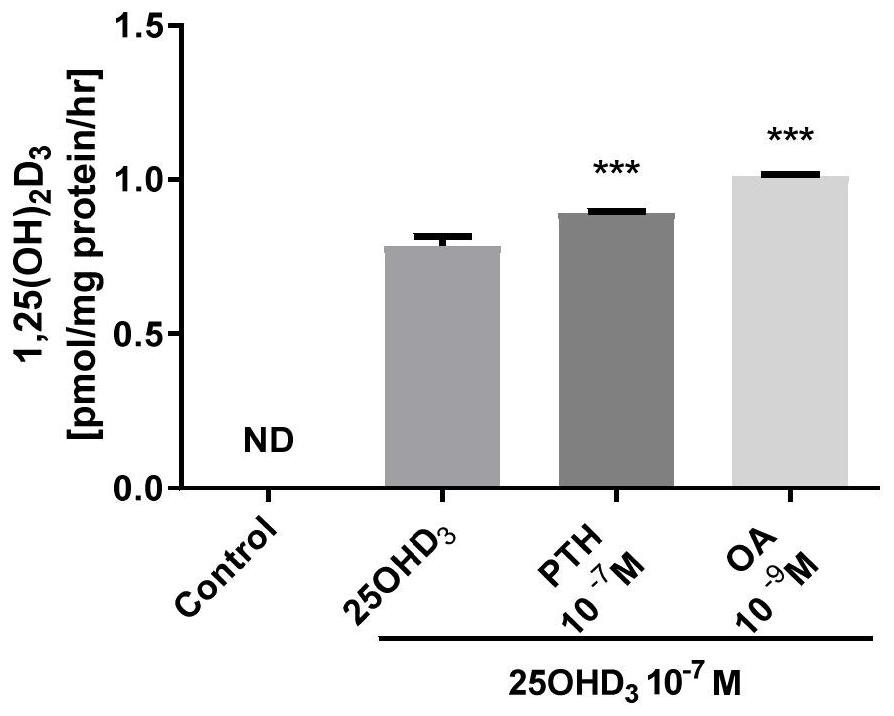
Vitamin D supplement preparation and its application
ActiveCN112386601BPromote absorptionSmall doseOrganic active ingredientsVitamin food ingredientsBone mineralBULK ACTIVE INGREDIENT
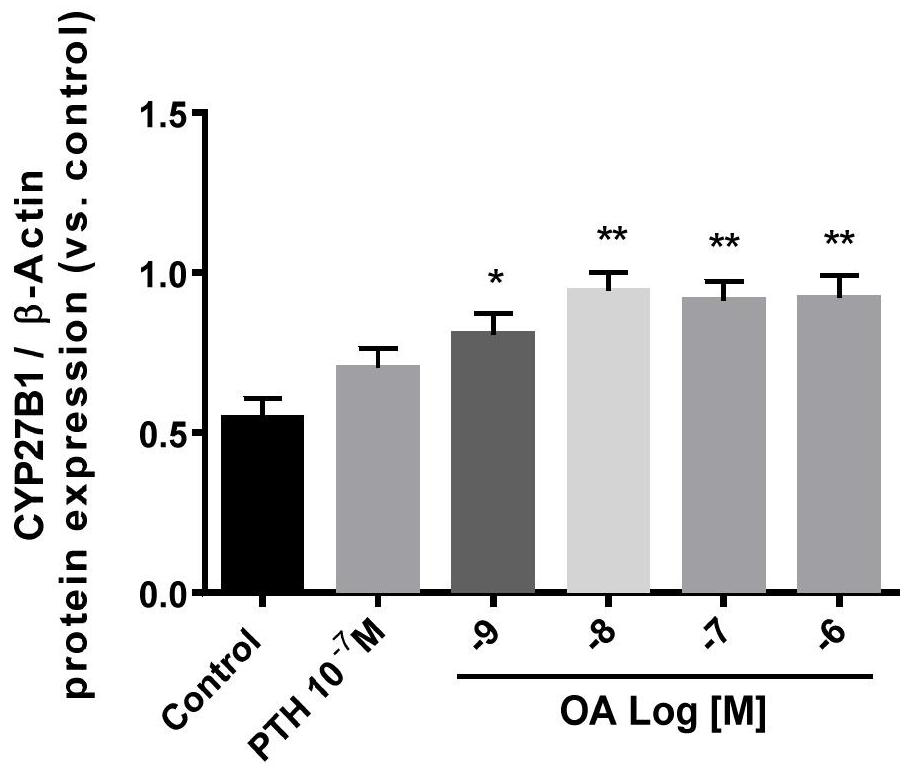
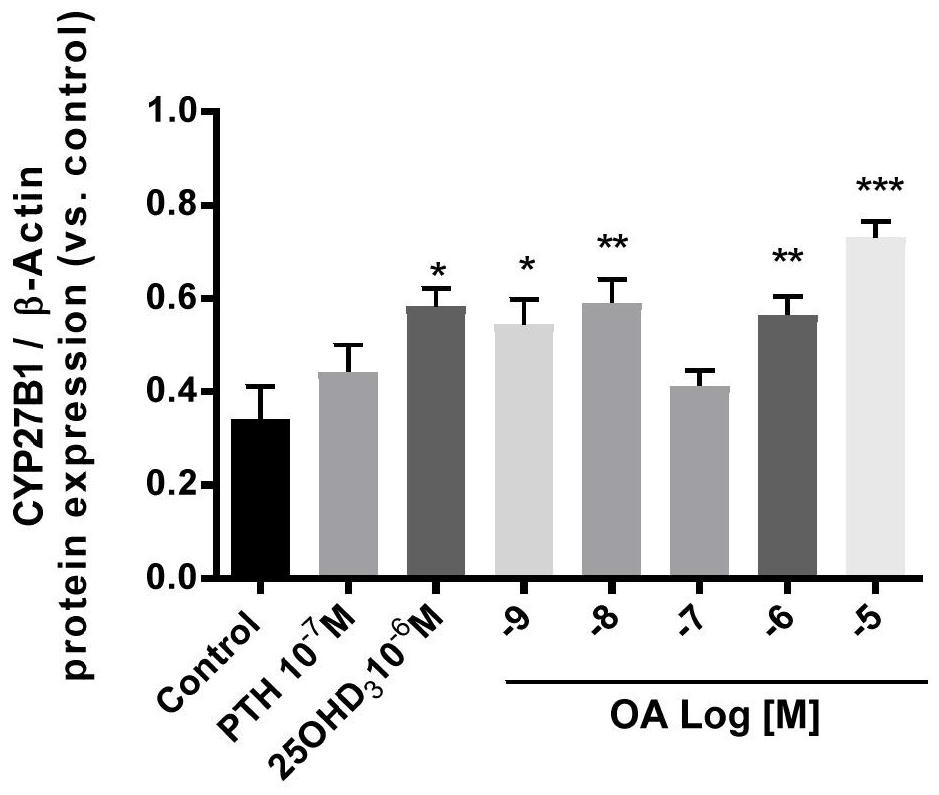
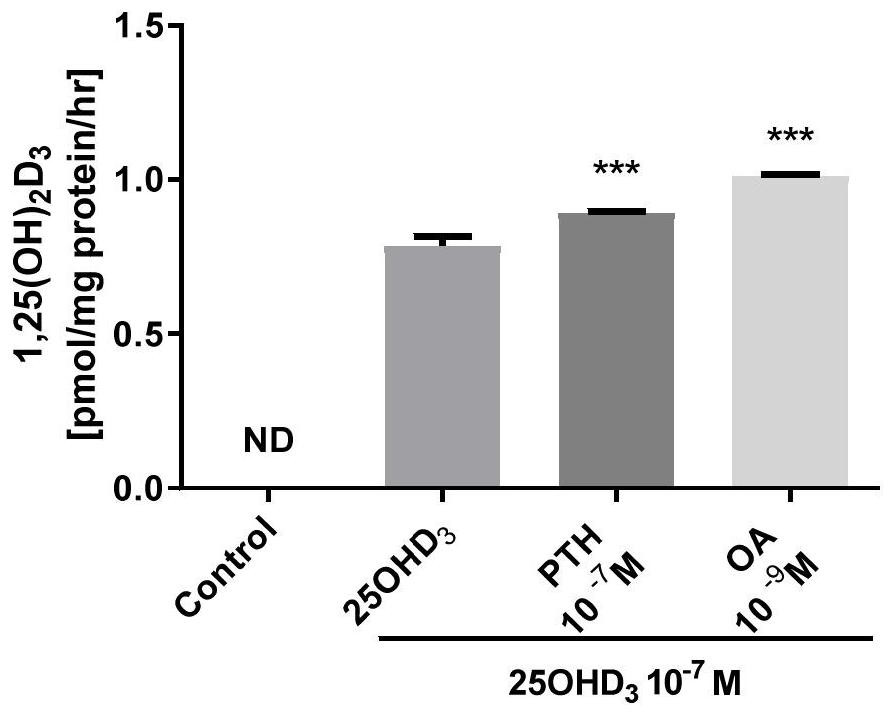
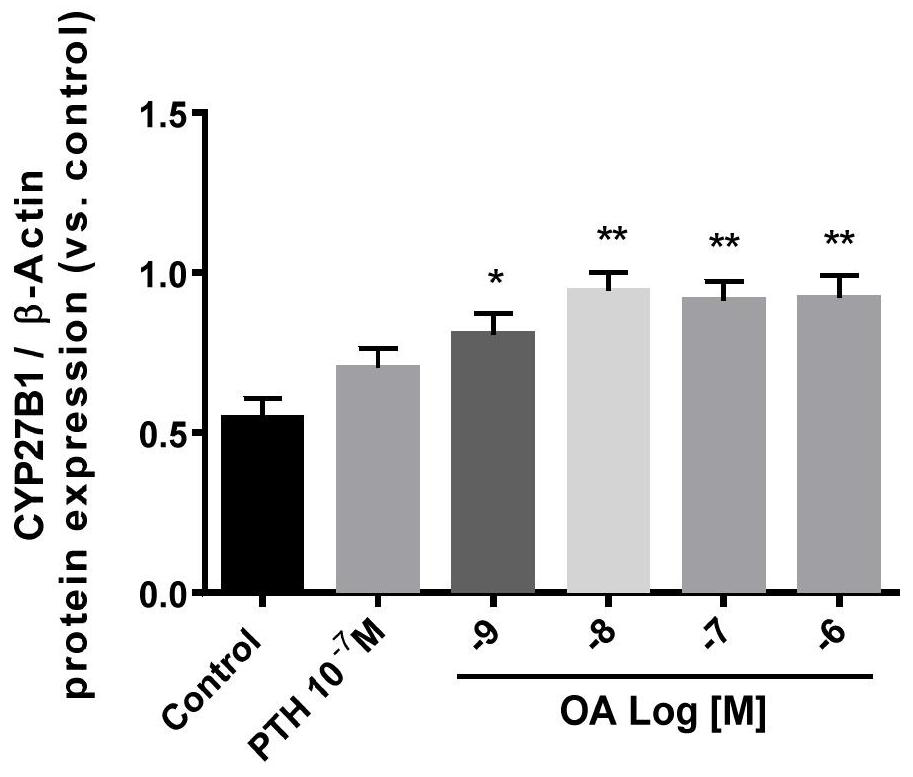
The invention belongs to the technical field of biomedicine, and in particular relates to a vitamin D supplement preparation and application thereof. The active ingredients of the vitamin D supplement preparation provided by the invention include: calcifediol and oleanolic acid. The synergistic effect of calcifediol and oleanolic acid can effectively promote the expression of CYP27B1 in bone marrow stem cells and osteoblasts, and increase the level of 1,25‑dihydroxyvitamin D 3 Synthesis of vitamin D, which can quickly supplement the vitamin D needed by the human body, especially in special populations 3 , regulate calcium and phosphorus metabolism, promote bone mineral deposition, and can be applied to the preparation of prevention or treatment of bone diseases caused by vitamin D deficiency.
Owner:THE HONG KONG POLYTECHNIC UNIV SHENZHEN RES INST
Autotrophic pseudonocardia and application thereof
The invention relates to autotrophic pseudonocardia and application thereof, and belongs to the technical field of microorganisms. The pseudonocardia autotrophicus can be used for preparing calcifediol and / or calcitriol, compared with an original strain, the yield is remarkably improved.
Owner:DONGGUAN HEC GENERIC DRUG R&D CO LTD +1
Medicine composition containing vitamin d and metformin
ActiveUS20140031322A1Effectively treat preventEffective treatmentBiocideDigestive systemDihydrotachysterolVitamina D2
A medicine composition contains vitamin D and metformin, wherein vitamin D comprises vitamin D2, vitamin D3, alphacalcidol, calcifediol, calcitriol, and dihydrotachysterol. The composition can be used in the preparation of a medicine for treating and / or preventing a polyp and cancer in large intestine.
Owner:GUANGDONG TAIHE MEDICINE SCI & TECH
Buffer composition for catalyzing the preparation of calcitriol or calcifediol and method for preparing calcitriol or calcifediol using same
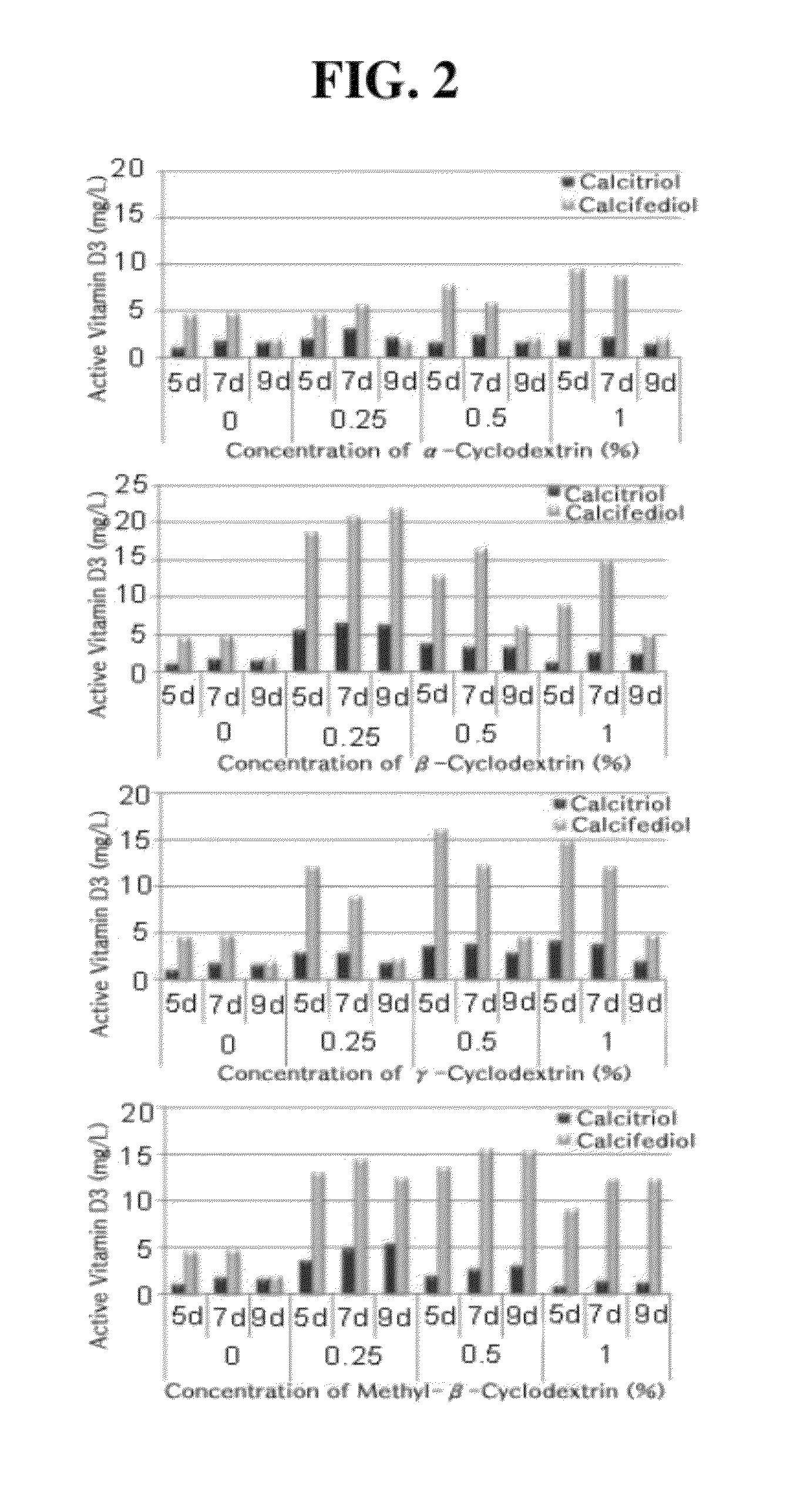
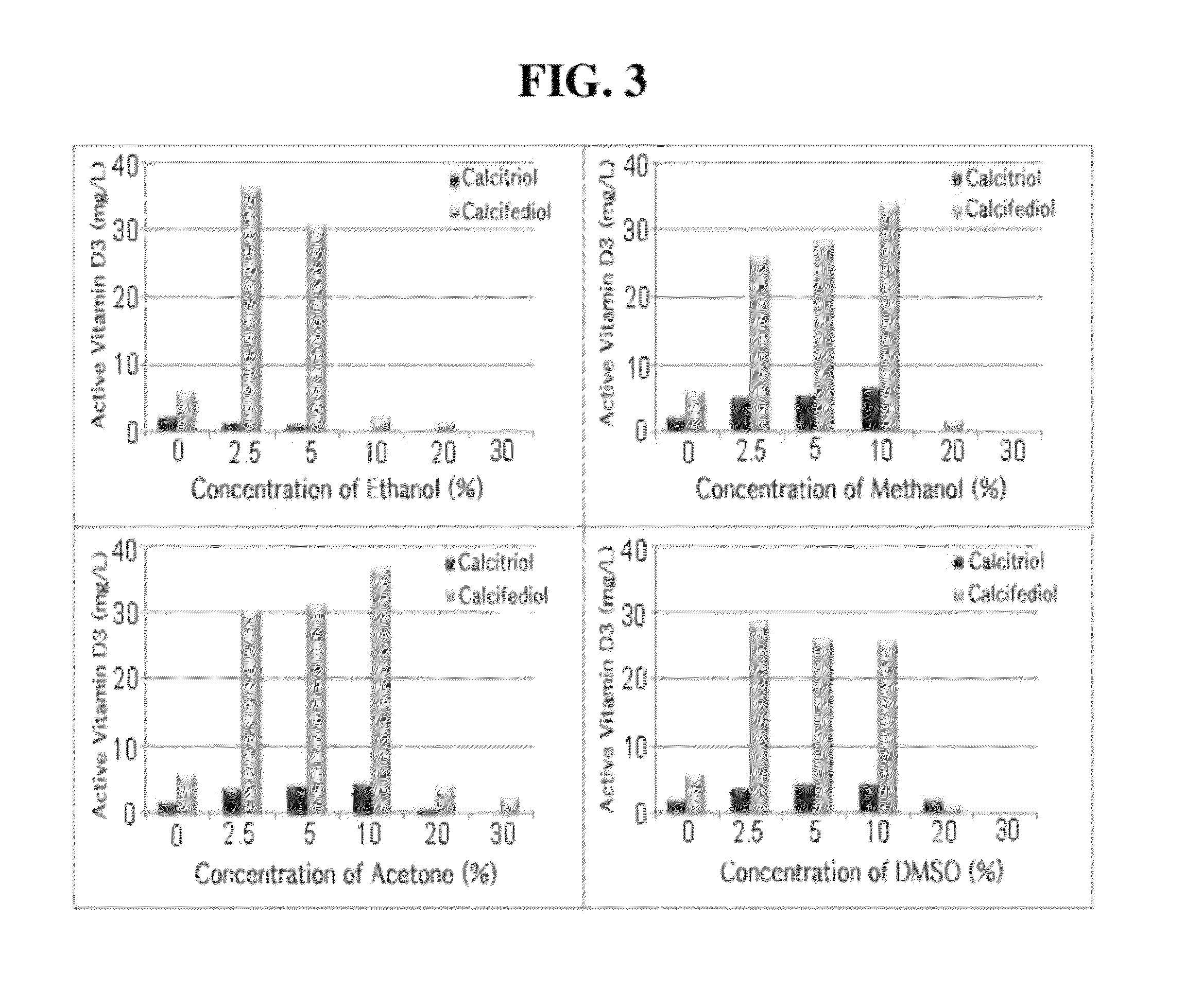
ActiveUS8530205B2Improve productivitySolution has disadvantageBacteriaHydrolasesCyclodextrinCalcitriol
The present invention relates to a buffer composition for promoting production of calcitriol or calcifediol, and a method for producing calcitriol or calcifediol using the same. More particularly, the present invention relates to a buffer composition for promoting production of calcitriol or calcifediol comprising a metallic compound, an organic solvent, cyclodextrin, tris(hydroxymethyl)aminomethane, sodium succinate, sodium chloride, magnesium chloride, and water, and a method for producing calcitriol or calcifediol using the same. In the method for producing calcitiriol or calcifediol, the production yield of calcitriol or calcifediol is high, and the bioconversion is carried out in an enzyme reaction system instead of in a microorganism culture system. Thus, it is not required to maintain a sterile state. Also, the separation / purification following the completion of a biocatalytic reaction can be carried out in a cleaner state than the microorganism culture method. Accordingly, there is an advantage in that a cost required for separation is low and the quality is improved. Furthermore, the buffer composition for promoting production of calcitriol or calcifediol can provide a high productivity of calcitriol or calcifediol.
Owner:IL DONG PHARMA CO LTD
Calcifediol and vitamin D3 eutectic crystal, preparation method and applications thereof
ActiveCN110128312AGood chemical stabilityImprove complianceOrganic active ingredientsMetabolism disorderSingle Crystal DiffractionX-ray
The invention relates to a calcifediol and vitamin D3 eutectic crystal, a preparation method and applications thereof, wherein a molar ratio of calcifediol to vitamin D3 in the eutectic crystal is 1:1, and the complete characterizing results of the calcifediol and vitamin D3 eutectic crystal by X-ray single crystal diffraction, X-ray powder diffraction, thermogravimetric analysis, differential thermal analysis, infrared spectral analysis and other methods show that the calcifediol and vitamin D3 eutectic crystal can significantly improve the chemical stability under illumination conditions; and the preparation method is simple, and can achieve repeated preparation.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Use of 25-hydroxy-vitamin d3 to affect human muscle physiology
InactiveUS20130150598A1Function increaseHigh strengthOrganic active ingredientsOrganic chemistryMuscle strengthPhysiology
The use of 25-OH D3 (calcifediol) to increase muscle strength, muscle function, or both is provided. Vitamin D3 (cholecalciferol) may optionally be used together with 25-OH D3. Forms and dosages of a pharmaceutical composition, as well as processes for manufacturing medicaments, are also disclosed.
Owner:DSM IP ASSETS BV
Feeding medium for calcifediol fermentation, feeding method for fermentation and fermentation method thereof
ActiveCN107365805BPromote proliferationGuaranteed Amination RequirementsBacteriaMicroorganism based processesBiotechnologyInorganic salts
The invention discloses a feeding medium for calcifediol fermentation, a feeding method for fermentation and a fermentation method thereof. The feeding medium includes: organic nitrogen source 0.2-3.0 g / 100ml, inorganic nitrogen source 0.2-3.0 g / 100ml, carbon source 0.5~3.0g / 100ml, inorganic salt A 0.1~1.0g / 100ml, inorganic salt B 0.1~1.0g / 100ml, trace element 0.0002~0.0006g / 100ml. The feeding method and the fermentation method include adding the feeding medium and water to the fermentation broth, which meets the nutritional requirements of calcifediol fermentation and satisfies the dissolved oxygen requirements of calcifediol fermentation, The power cost is reduced and the fermentation efficiency is improved.
Owner:太原市威尔潞威科技发展有限公司 +1
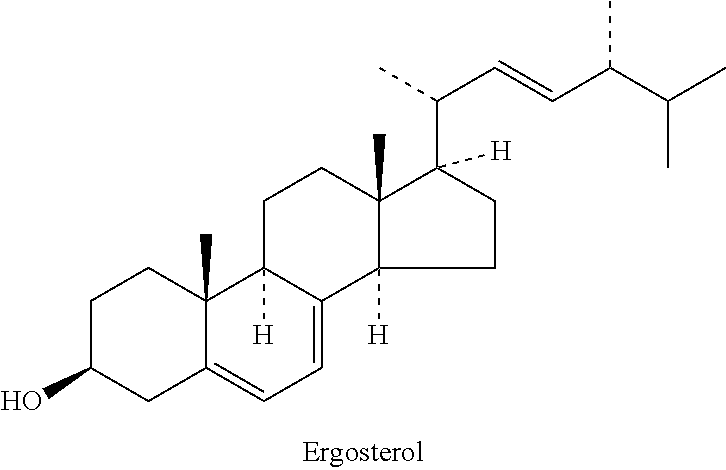
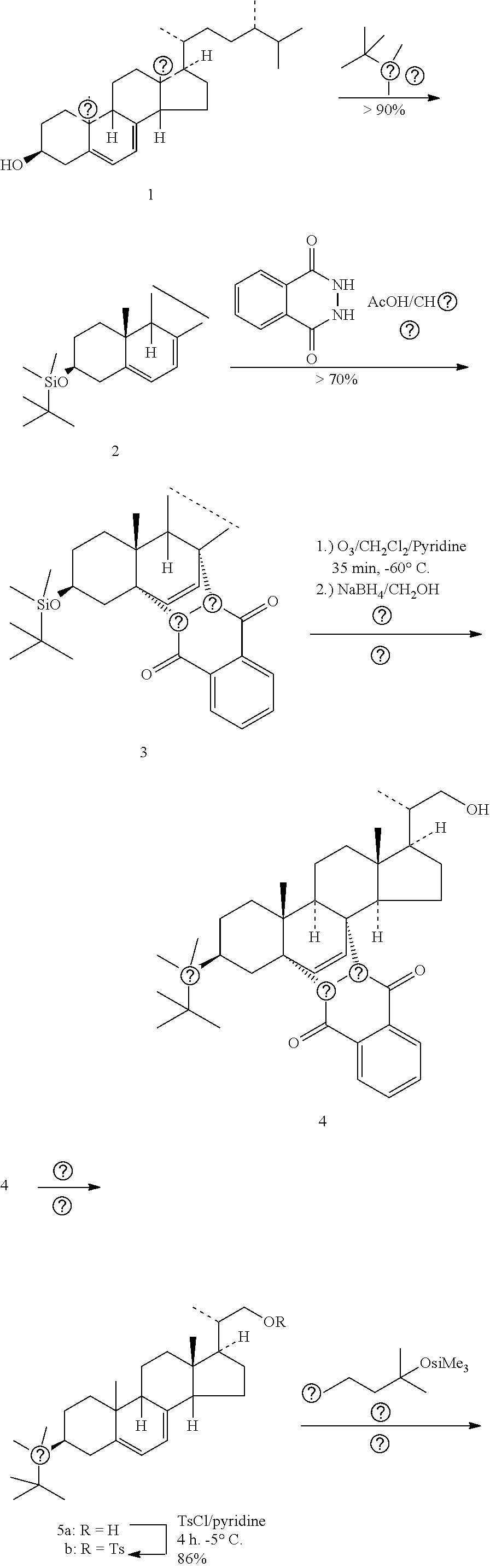
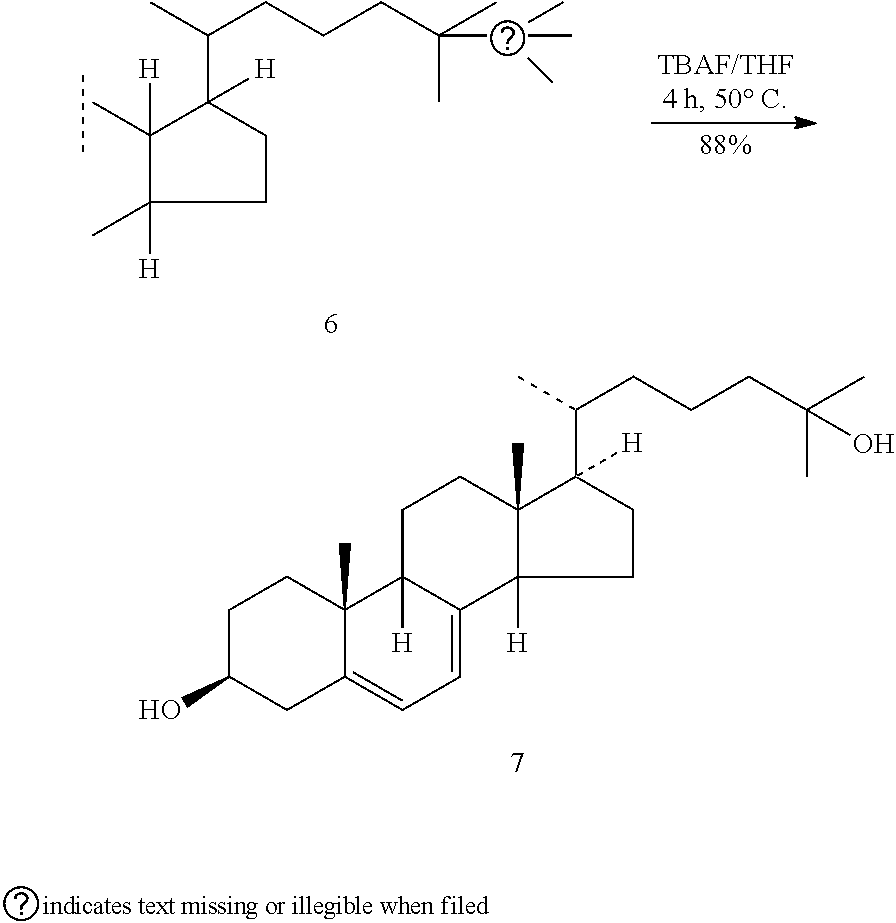
Improved, cost effective process for synthesis of vitamin d3 and its analogue calcifediol from ergosterol
PendingUS20220267266A1Increased riskMinimizing side-productsGroup 4/14 element organic compoundsSteroidsErgostanolBiochemical engineering
Disclosed herein is an improved and efficient process for synthesis of vitamin D3 and its analogue Calcifediol from Ergosterol. Particularly, the present invention discloses the synthesis of key intermediate 3β-tert-Butyldimethylsilyloxy-22-hydroxy-23,24-bisnorchola-5,7-diene (5), and novel intermediate β-tert-Butyldimethylsilyloxy-22-iodo-23,24-bisnorchola-5,7-diene (9) by a simple and cost effective process. The industrially viable processes for preparation of said intermediate(s) results in providing provitamins with various side chains and the desired products in high yield.
Owner:FERMENTA BIOTECH
Fusion protein or variant thereof and application of fusion protein or variant thereof in preparation of calcifediol
PendingCN113583983AImprove electron transfer efficiencyIncrease productionBacteriaAntibody mimetics/scaffoldsElectron transferCalcifediol
The invention provides a fusion protein or a variant thereof, the fusion protein comprises K1 and RhFR, the amino acid sequence of the K1 is as shown in SEQ ID NO: 1, and the amino acid sequence of the RhFR is as shown in amino acids from the 466th site to the 773rd site of SEQ ID NO:5. The invention also provides a preparation method and application of the fusion protein or the variant thereof. When the fusion protein or the variant of the fusion protein is applied to catalysis of VD3 to synthesize 25-hydroxyvitamin D3 (calcifediol), no extra electron transfer related protein needs to be added, the operation is simple and convenient, the electron transfer efficiency in the fusion protein is high, the catalytic efficiency is high, the yield of the obtained calcifediol is remarkably improved, the production cost is reduced, and the fusion protein or the variant of the fusion protein is suitable for industrial production.
Owner:ABIOCHEM BIOTECH CO LTD
Treating conditions associated with increased eotaxin with 25-hydroxyvitamin d3
InactiveUS20130252927A1Symptoms improvedReduce the amount requiredBiocideOrganic active ingredientsEosinophil Chemotactic FactorsMedicine
The present invention relates to treating / preventing conditions associated with an increased level of eotaxin in a human with 25-hydroxyvitamin D3 (calcifediol). Optionally, vitamin D3 may be used together with 25-hydroxyvitamin D3.
Owner:DSM IP ASSETS BV
Combination of vitamin d and 25-hydroxyvitamin d 3
We disclose compositions comprising Vitamin D (cholecalciferol and / or ergocalciferol) and 25-OH D3 (calcifediol), and use of those compositions to affect at least concentration, bioavailability, metabolism, or efficacy of vitamin D in a human. Forms and dosages of the composition, as well as processes for manufacturing a spray-dried formulation, are also disclosed.
Owner:BUCK NEIL ROBERT +5
Genetically engineered bacterium and application thereof
PendingCN113493756AIncrease productionMeet the needs of industrial productionBacteriaMicroorganism based processesMicrobiologyEngineered genetic
The invention discloses a genetically engineered bacterium. The genetically engineered bacterium is an engineered bacterium constructed by expressing a CYP gene, a ferredox protein gene, a ferredox protein reductase gene and a dehydrogenase gene in a cell; wherein an amino acid sequence coded by the CYP gene is as shown in SEQ ID NO. 1, SEQ ID NO. 3, SEQ ID NO. 9 or SEQ ID NO. 11. The invention further discloses application of the genetically engineered bacterium in preparation of calcifediol and / or calcitriol. When the engineering bacterium of the invention is applied to synthesis of the calcifediol and the calcitriol, the yield is remarkably increased, and when the engineering bacterium is applied to industrial production, the cost is lower, the reaction specificity is high, the reaction condition is mild, the engineering bacterium is environmentally friendly, and the industrial production requirements of the calcifediol and the calcitriol are met.
Owner:弈柯莱生物科技(集团)股份有限公司
Vitamin D supplementing preparation and application thereof
ActiveCN112386601APromote absorptionSmall doseOrganic active ingredientsVitamin food ingredientsBone mineralBULK ACTIVE INGREDIENT
The invention belongs to the technical field of biological medicine, and particularly relates to a vitamin D supplementing preparation and application thereof. The vitamin D supplementing preparationcomprises the following active ingredients: calcifediol and oleanolic acid. The calcitriol and the oleanolic acid have a synergistic effect, so that bone marrow stem cells and osteoblasts can be effectively promoted to express CYP27B1, the synthesis of 1,25-dihydroxy vitamin D3 is improved, vitamin D3 required by human bodies, particularly special crowds, can be quickly supplemented, calcium and phosphorus metabolism can be regulated, bone mineral deposition can be promoted, and the vitamin D supplementing preparation can be applied to preparation of medicines for preventing or treating bone diseases caused by vitamin D deficiency.
Owner:THE HONG KONG POLYTECHNIC UNIV SHENZHEN RES INST
Medicine composition containing vitamin D and metformin
ActiveUS9333210B2Effective treatmentReduce in quantityDigestive systemAntineoplastic agentsDihydrotachysterolVitamina D2
A medicine composition contains vitamin D and metformin, wherein vitamin D comprises vitamin D2, vitamin D3, alphacalcidol, calcifediol, calcitriol, and dihydrotachysterol. The composition can be used in the preparation of a medicine for treating and / or preventing a polyp and cancer in large intestine.
Owner:GUANGDONG TAIHE MEDICINE SCI & TECH
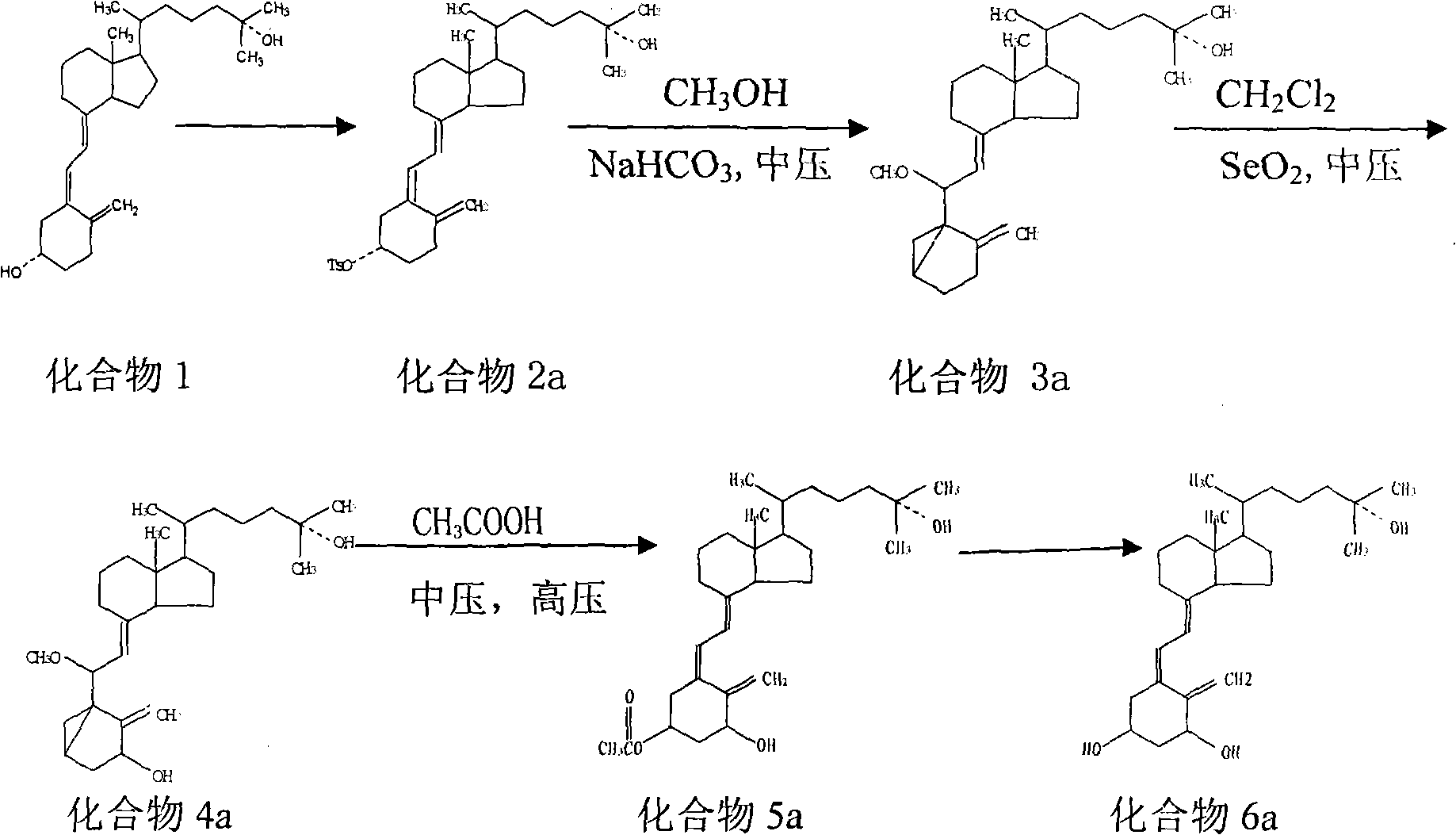
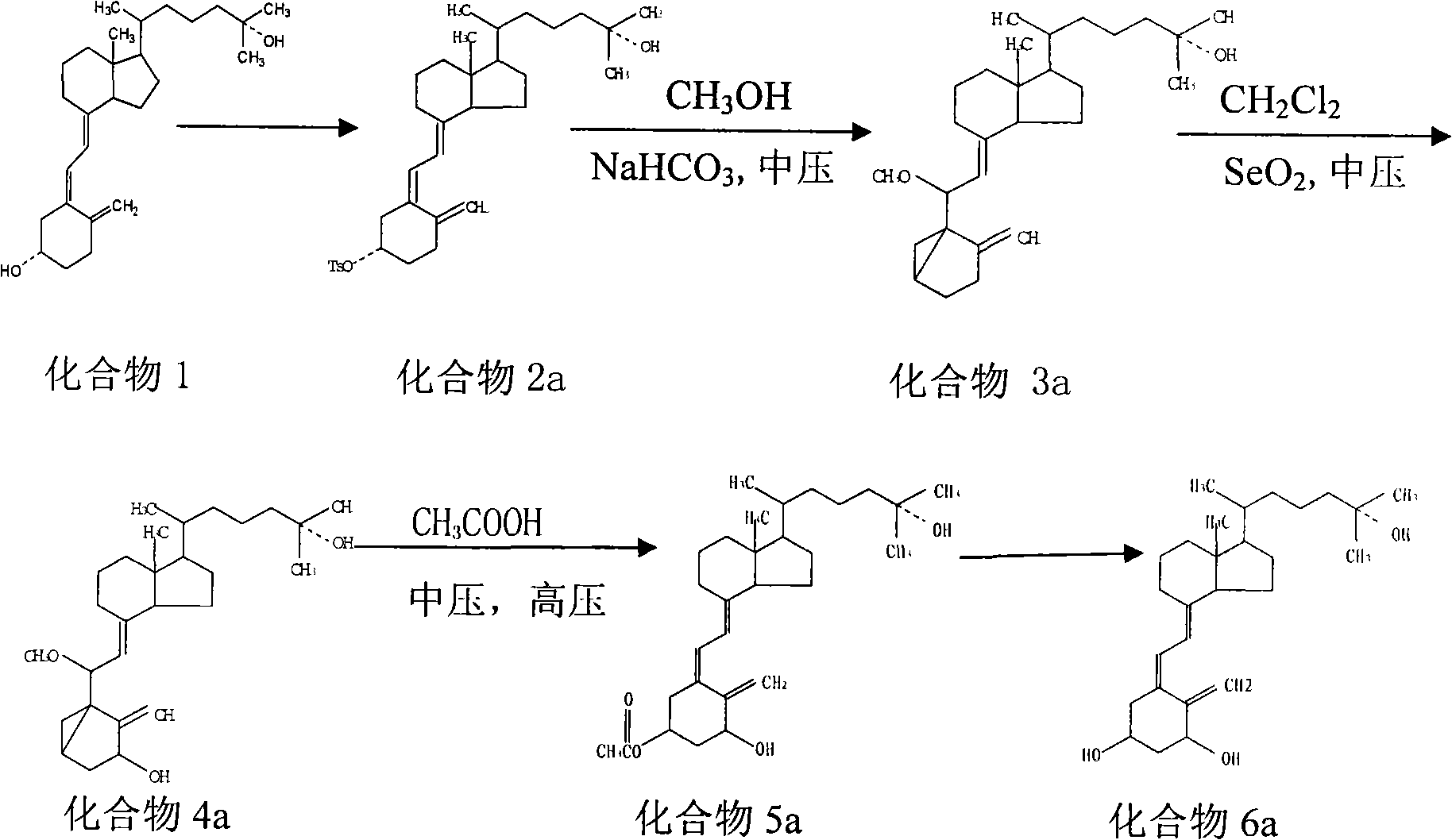
Preparation method of calcifediol intermediate A ring
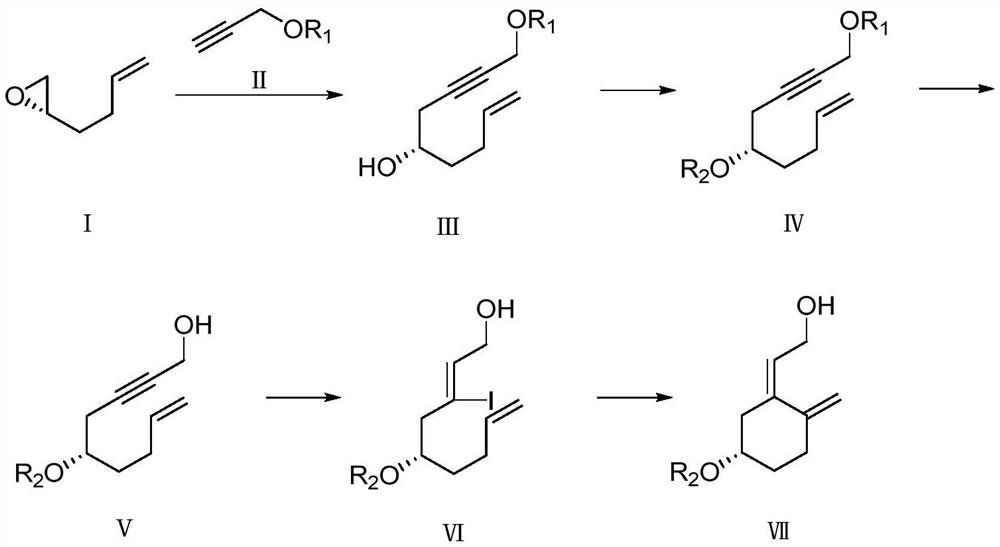
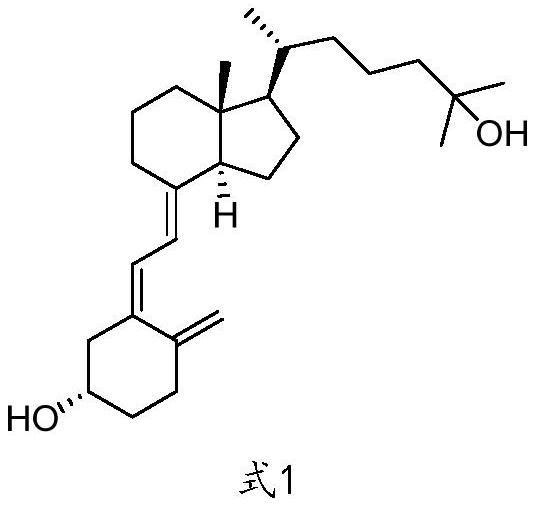
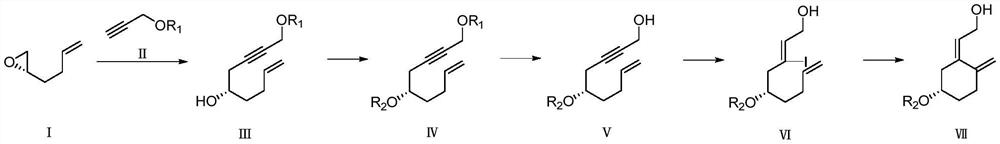
InactiveCN113651845AWide variety of sourcesReduce pollutionGroup 4/14 element organic compoundsOrganic compound preparationChemical reactionBiochemical engineering
The invention discloses a preparation method of a calcifediol intermediate ring A, and belongs to the technical field of organic chemistry. A compound III, a compound IV and a compound V are respectively obtained by taking a compound I as an initial raw material according to a synthesis route through a process shown in a reaction formula, and a key intermediate VII is finally obtained. The synthesis process adopts conventional organic chemical reactions, the raw materials are low in cost and easy to obtain, the process is simple, the synthesis route is short, the yield is high, the product quality is stable, large-scale production is easily achieved, and the method has good economic benefits and wide application prospects.
Owner:甘肃皓天医药科技有限责任公司
A novel separation and purification method of calcifediol (25-hydroxyvitamin d3)
A novel calcifediol (25-hydroxyvitamin D3) separation and purification method is provided. The method mainly includes subjecting calcifediol to extraction and separation with an organic solvent; concentrating an extract liquid; dissolving the concentrate again to obtain a calcifediol stock solution for column chromatography; preliminarily purifying the calcifediol product by utilizing a gel chromatographic column; then further purifying and separating the calcifediol product by utilizing macroporous adsorption resin; finally preparing a high-purity calcifediol product through column chromatography on silica gel; subjecting an eluate to concentration and crystallization to obtain a calcifediol crystal product. The method is advantageous in that 1) the calcifediol product obtained by separation and purification through the method is high in purity and high in yield; 2) gel column chromatography and macroporous resin adsorption chromatography are applied for separation and purification ofthe calcifediol product for the first time; 3) the method is simple in process and convenient to operate, a plurality of times of cyclic operation can be performed, the equipment cost is low and themethod is particularly suitable for industrial promotion; 4) organic solvents adopted in the method can be recycled and reused, so that the production cost is reduced, and the method is economical andenvironmentally friendly.
Owner:山东惠仕莱生物科技有限公司
Drug combination for use in the treatment of inflammatory diseases
PendingUS20220323380A1Eliminate generationAvoid it happening againOrganic active ingredientsNervous disorderVitamin K2Disease
The present invention is inter alia concerned with a combination (i) a first compound selected from the group consisting of a norepinephrine-dopamine reuptake inhibitor (NDRI), a catecholamine and pharmaceutically acceptable salts thereof, (ii) a second compound selected from the group consisting of vitamin D3, calcifediol, calcitriol, vitamin D2, ercalcidiol and ercalcitriol, and (iii) a third compound selected from the group consisting of vitamin K1, vitamin K2 and vitamin K3, for use in the treatment of an inflammatory disease, preferably multiple sclerosis.
Owner:ISANAS AG
Method of controlling progression of hyperparathyroidism with calcifediol, and compositions for use therein
Owner:EIRGEN PHARMA LTD
Novel method for synthesizing 25-oh cholesterol/calcifediol from phytosterol
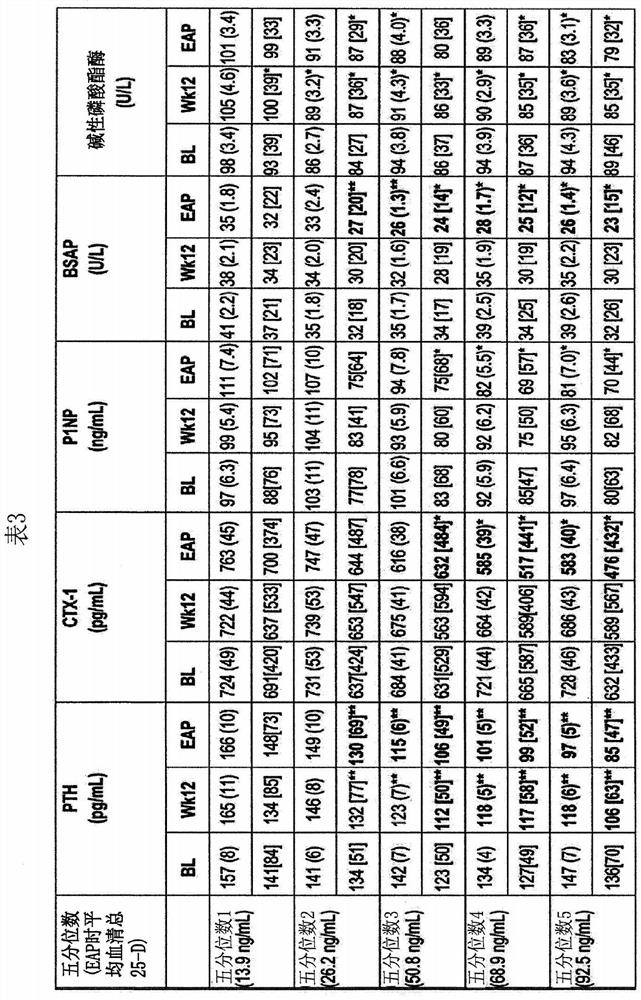
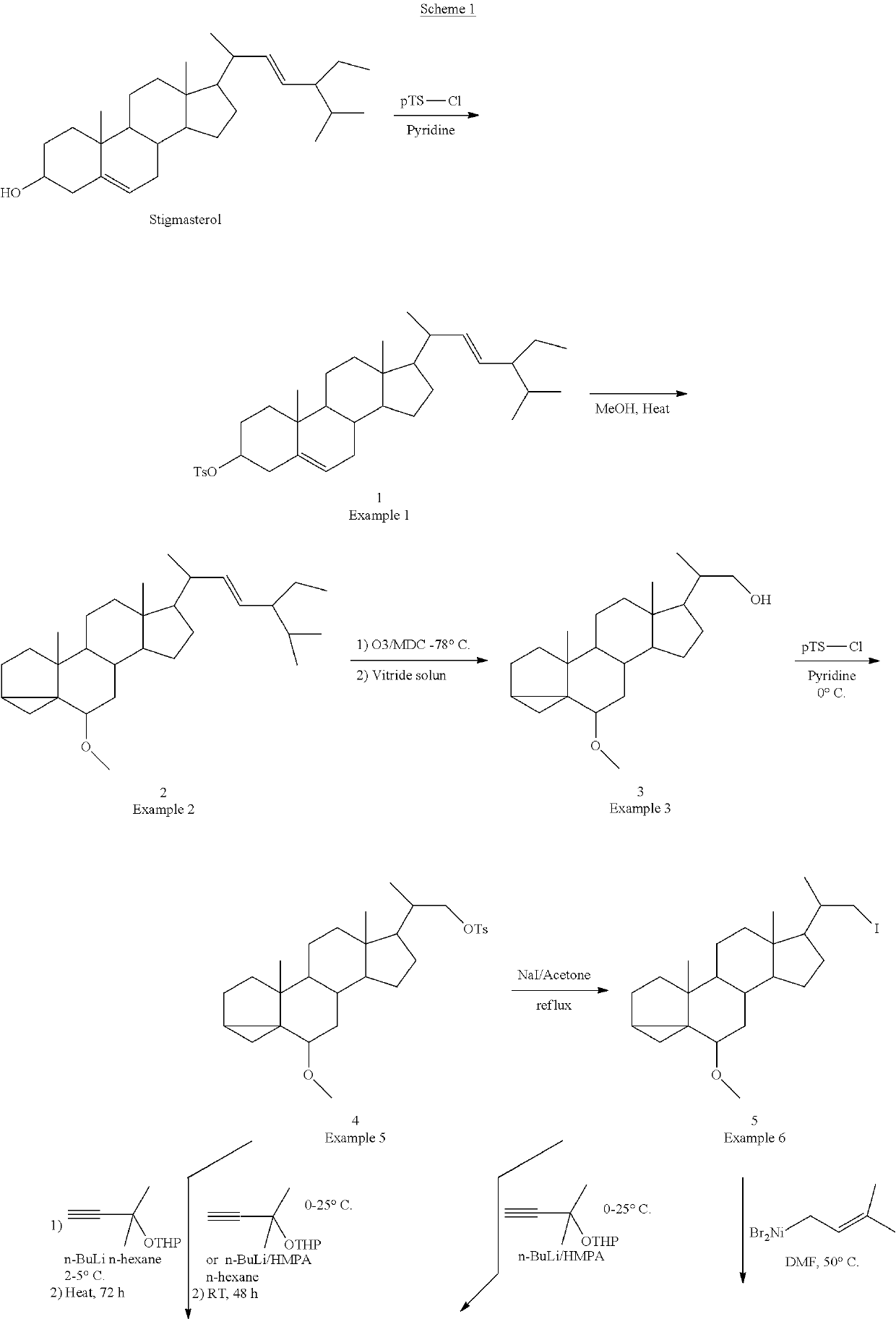
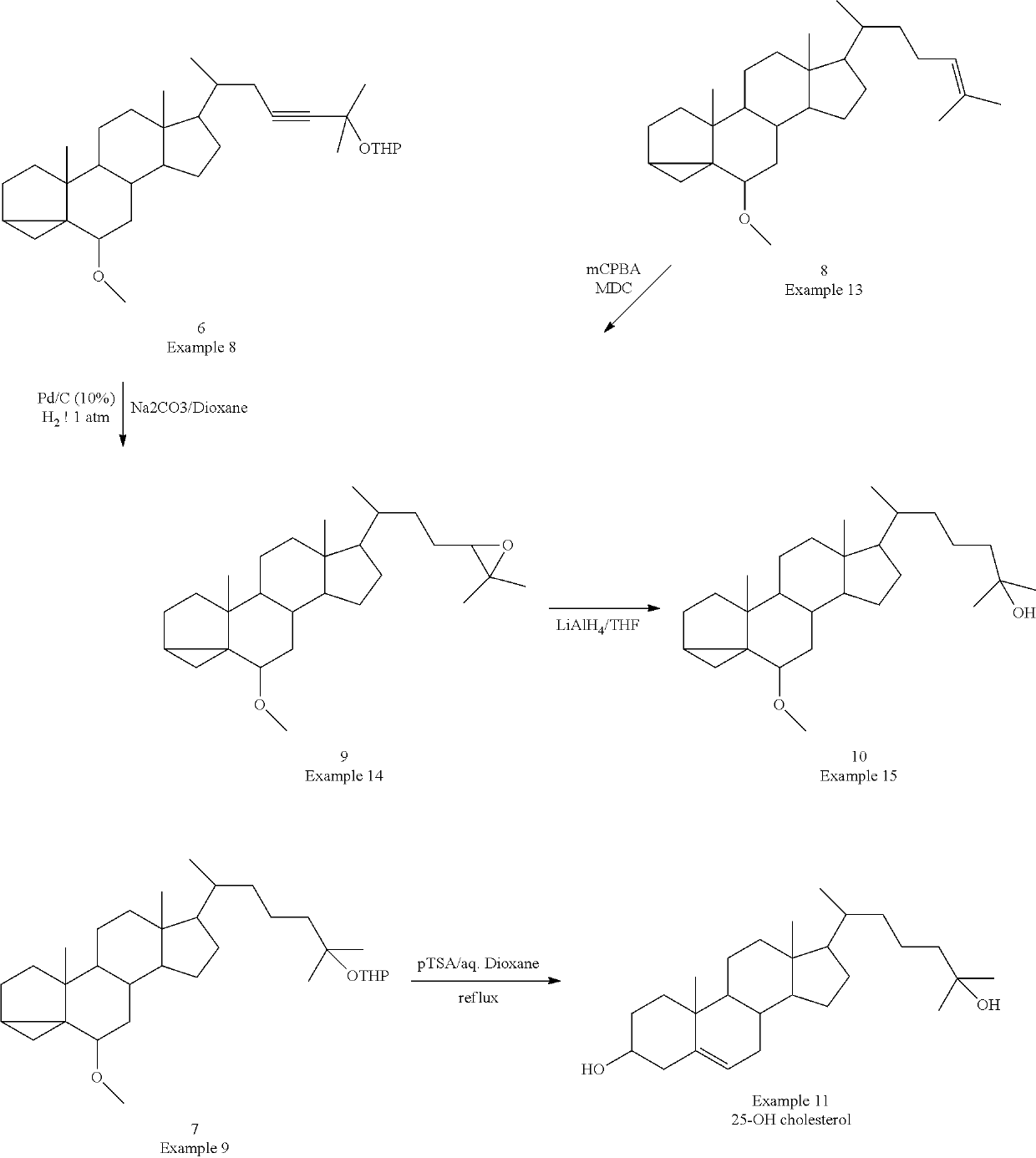
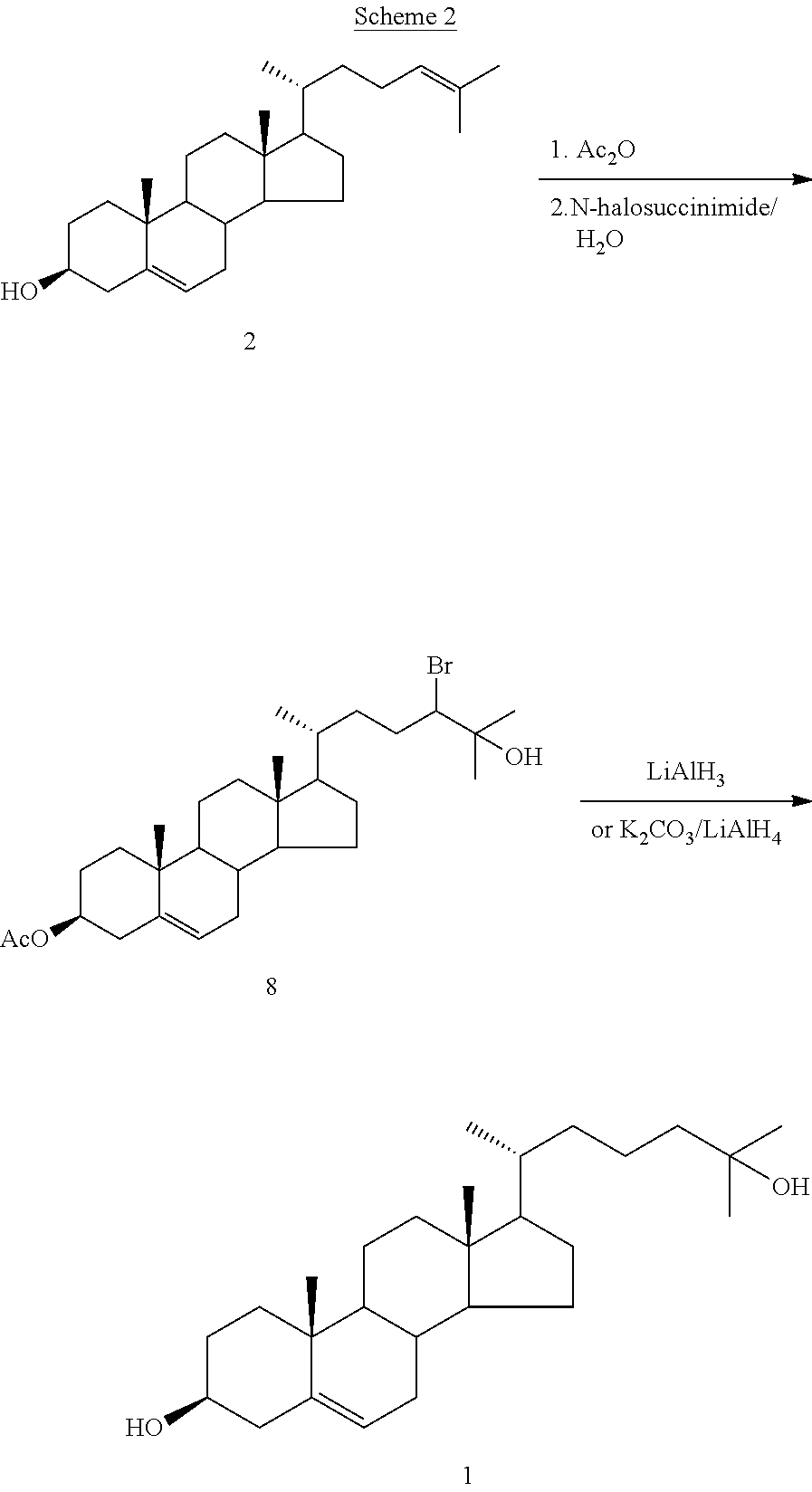
The present invention discloses novel method for synthesizing vegan 25-OH cholesterol / Calcifediol from inexpensive crude phytosterol. According to the method, Phytosterols are reacted to form corresponding i-steroid through tosylation and methanolysis. i-steroid on reductive ozonolysis to C-22 alcohol and conversion via C-22 tosylate to C-22 iodide in good yield. Coupling of C-22 tosylate with Grignard reagent of 4-bromo-2-methyl-2-[(trimethylsilyl)oxy]butane followed by deprotection yielded 25-OH cholesterol. In a process variant, nickel mediated conjugate addition of C-22 iodide to an electron deficient alkene ethyl acrylate and treating corresponding ester with methyl magnesium bromide as means of installing the side chain of 25-OH cholesterol in high yield. Further bromination reaction of 25-OH cholesterol diacetate followed by dehydrobromination using TBAF yielded 25-OH 7-dehydrocholesterol. Further photo reaction of 25-OH 7-dehydrocholesterol in to previtamin D3 using high or medium pressure mercury lamp and subsequent thermal reaction of previtamin D3 to 25-OH vitamin D3 (Calcifediol) in good yield.
Owner:FERMENTA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com