A novel separation and purification method of calcifediol (25-hydroxyvitamin d3)
A technology of hydroxyvitamins and calcifediol, which is applied in the field of bioengineering, can solve the problems of less description of the extraction and purification process of calcifediol, difficulty in industrial scale-up production, and high cost of equipment, so as to achieve strong repeatable operation and high equipment cost. The effect of low cost and large processing volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
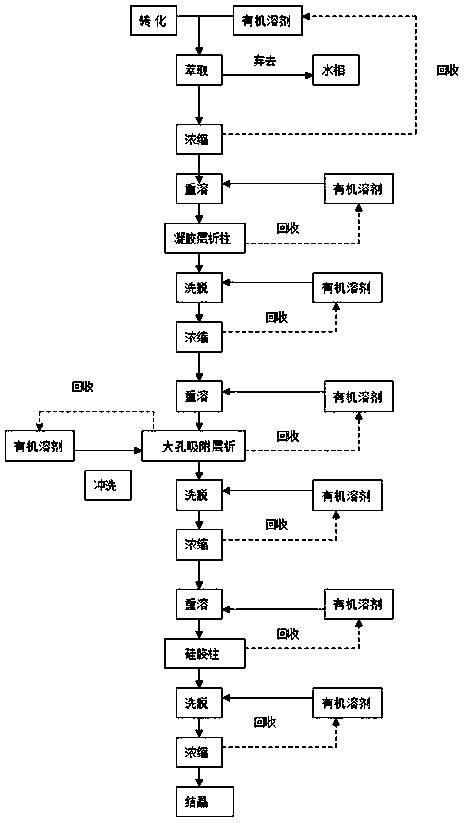
[0032] (1) Vitamin D is catalyzed by microbial enzymes 3 Generate calcifediol (25-hydroxyvitamin D 3 ) into the conversion liquid, add ethyl acetate according to the volume ratio of 1:1, shake and extract for 1 h, collect the organic solvent in the upper layer, and obtain the calcifediol extract a.
[0033] (2) Concentrate the above calcifediol extract a under reduced pressure, recover ethyl acetate, redissolve the concentrate with methanol, adjust the concentration of calcifediol to 0.5 mg / L, and obtain column solution b.
[0034] (3) After the agarose gel filtration chromatography is activated, the column is packed by a wet method, and the upper column solution b is passed through the agarose gel filtration chromatography column at a flow rate of 2BV / h, and the chromatographic molecular weight is selected to be 400Da. 1BV of ethyl acetate was used as the eluent, and it was eluted at a flow rate of 2BV / h. The eluate was collected, concentrated under reduced pressure at 60°C ...
Embodiment 2
[0036] (1) Vitamin D is catalyzed by microbial enzymes 3 Generate calcifediol (25-hydroxyvitamin D 3 ) into the transformation liquid, add ethyl hexanoate at a volume ratio of 1:2, shake and extract for 0.5 h, collect the organic solvent in the upper layer, and obtain calcifediol extract a.
[0037] (2) Concentrate the above calcifediol extract a under reduced pressure, recover ethyl caproate, redissolve the concentrate with methanol, adjust the concentration of calcifediol to 0.8 mg / L, and obtain column solution b.
[0038] (3) After the dextran gel filtration chromatography is activated, the column is packed by a wet method, and the upper column solution b is passed through the dextran gel filtration chromatography column at a flow rate of 2BV / h, and the chromatography molecular weight is selected as 500Da, using 1BV of ethyl acetate as the eluent, eluted it at a flow rate of 2BV / h, and collected the eluate.
[0039] (4), the above-mentioned eluent was concentrated under r...
Embodiment 3
[0042] (1) Vitamin D is catalyzed by microbial enzymes3 Generate calcifediol (25-hydroxyvitamin D 3 ) into the transformation solution, petroleum ether was added at a volume ratio of 1:5, extracted by shaking for 0.5 h, and the organic solvent in the upper layer was collected to obtain calcifediol extract a.
[0043] (2) Concentrate the above calcifediol extract a under reduced pressure, recover petroleum ether, redissolve the concentrate with methanol, adjust the concentration of calcifediol to 1 mg / L, and obtain column solution b.
[0044] (3) After the agarose gel filtration chromatography is activated, the column is packed by a wet method, and the upper column solution b is passed through the agarose gel filtration chromatography column at a flow rate of 2BV / h, and the molecular weight of the chromatography is selected to be 700Da. 1BV of ethyl acetate was used as the eluent, and it was eluted at a flow rate of 2BV / h, and the eluate was collected.
[0045] (4), the above-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com