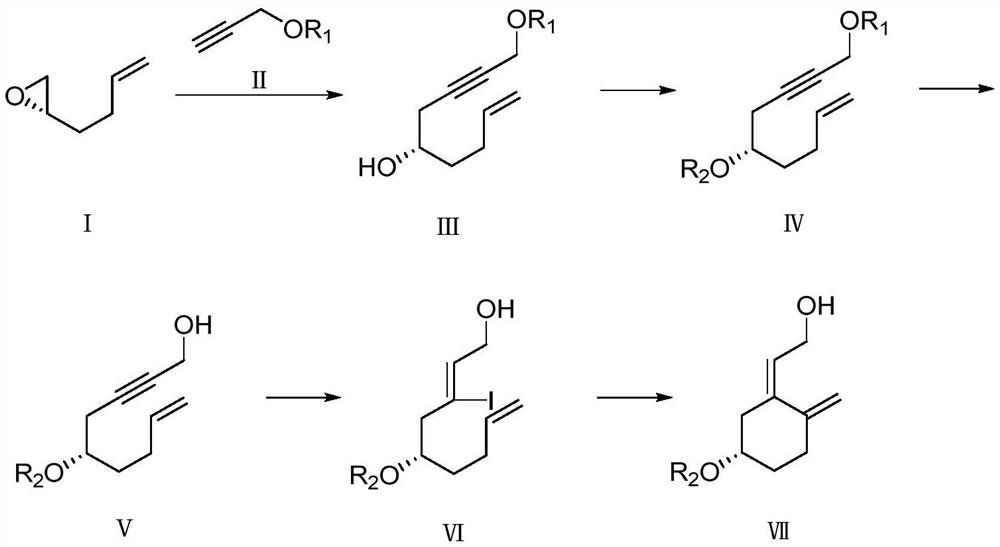
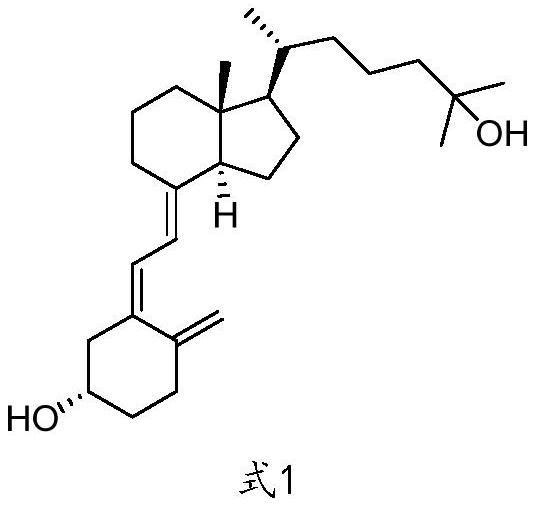
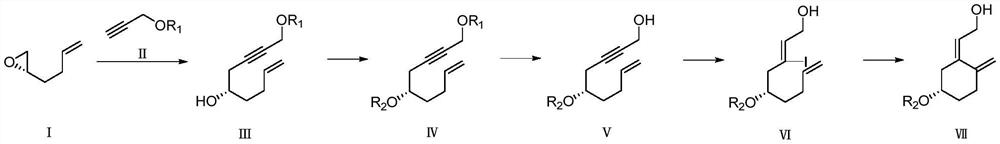
Preparation method of calcifediol intermediate A ring
A technology for calcifediol and intermediates, which is applied in the field of preparation of the A-ring of calcifediol intermediates, can solve the problems of long synthesis routes, difficulty in realizing mass production, etc., and achieves a short synthesis route, simple operation and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of calcifediol intermediate A ring, the synthetic route is described in the following formula:
[0040]
[0041] The specific preparation method is:
[0042] Preparation of compound Ⅲ-1
[0043] In the synthetic route shown in step 1, the solvent is tetrahydrofuran.
[0044] Under argon protection, compound II-1 (269.00g, 1.53mol) was dissolved in 1000mL tetrahydrofuran in a 2000mL three-neck flask, cooled to -70°C, and n-butyllithium (611.50ml, 1.53mol) was added dropwise, and stirred for 60min. Add compound I (50.00 g, 0.51 mol), and then add boron trifluoride ether (80.00 g, 0.56 mol), and continue stirring for 60 min. The reaction was quenched by adding saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, dried and concentrated to obtain a crude product, which was subjected to silica gel column chromatography to obtain Compound III-1 (120 g) with a yield of 85.7%.
[0045] Preparation of compound Ⅳ-1
[0046] Under...
Embodiment 2
[0054] A preparation method of calcifediol intermediate A ring, the synthetic route is described in the following formula:
[0055]
[0056] The specific preparation method is:
[0057] Preparation of compound Ⅲ-2
[0058] Under the protection of argon, compound II-2 (257.04g, 1.84mol) was dissolved in 1000mL ether in a 2000mL three-necked flask, cooled to -78°C, and n-butyllithium (736.00ml, 1.84mol) was added dropwise, and stirred for 50min. Add compound I (60.00 g, 0.61 mol), and then add boron trifluoride ether (86.90 g, 0.61 mol), and stir for 60 min. Saturated aqueous sodium bicarbonate solution was added, the organic phase was separated, dried and concentrated to obtain a crude product, which was subjected to silica gel column chromatography to obtain Compound III-2 (120.50 g), with a yield of 82.8%.
[0059] Preparation of compound Ⅳ-2
[0060] Under the protection of argon, compound III-2 (120.00g, 0.50mol) was dissolved in 1200mL dichloromethane in a 2000mL thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com