Method of controlling progression of hyperparathyroidism with calcifediol, and compositions for use therein
A technology for patients and hydroxyvitamins, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as lack of consensus on definitions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
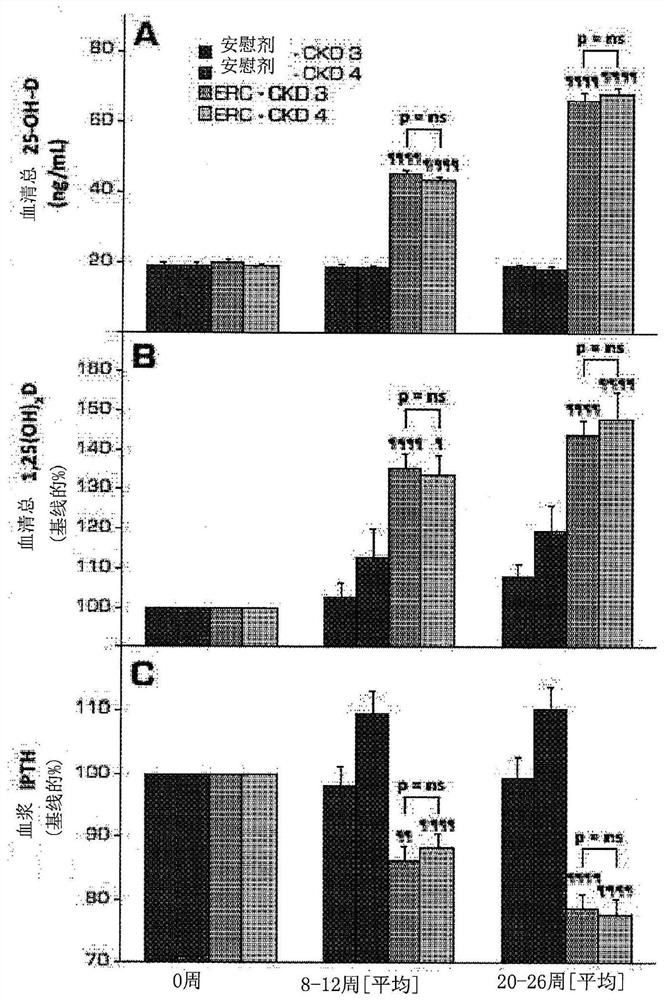
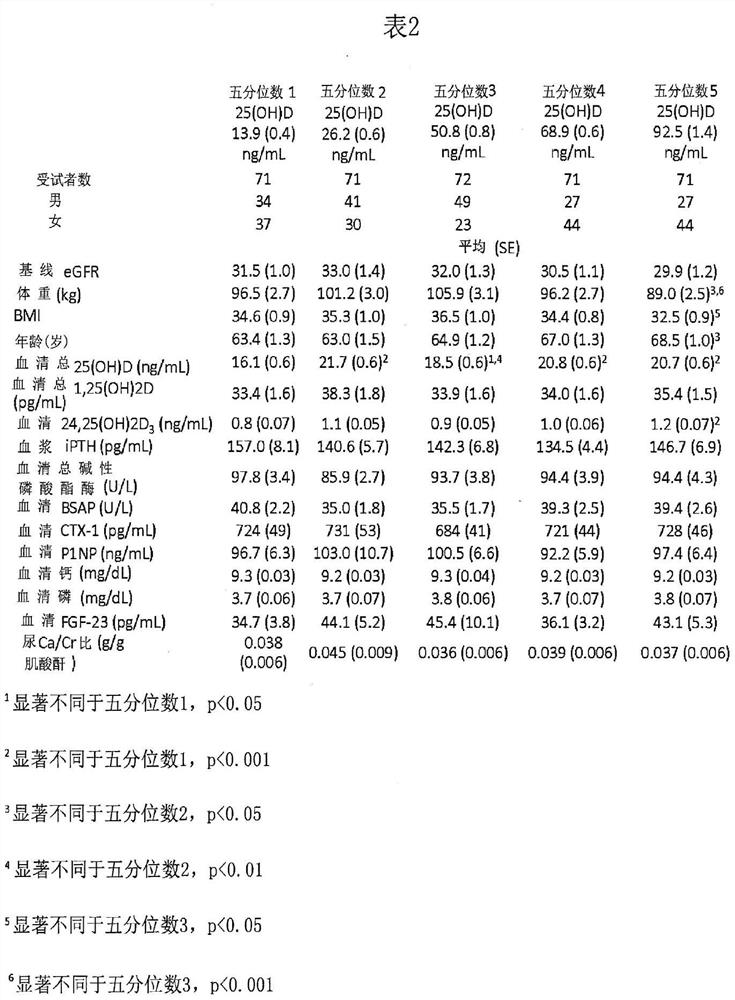
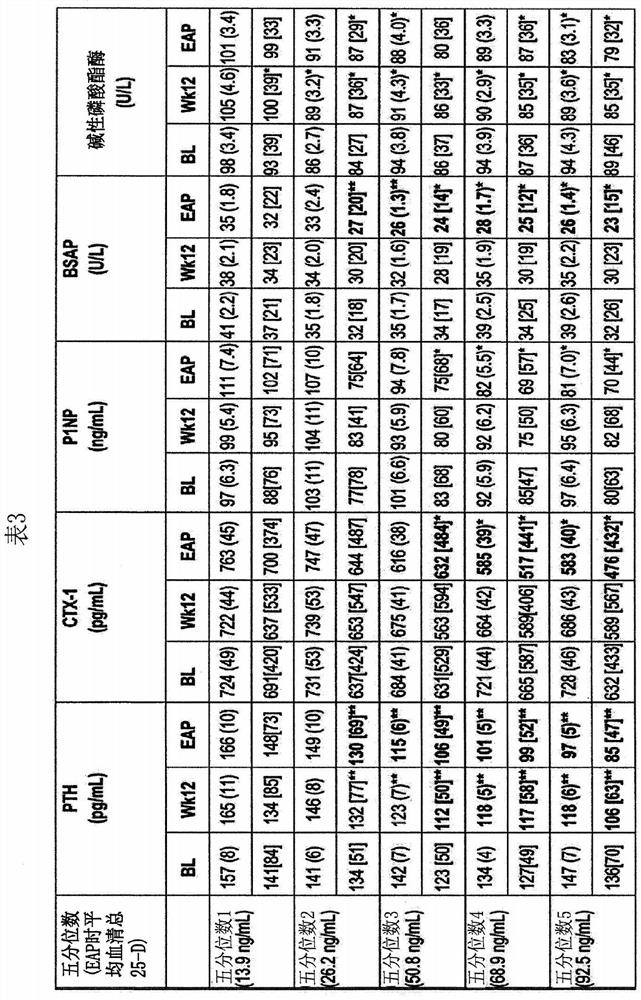
[0100] In two identical, parallel, randomized, double-blind studies, adult subjects (n=429) with SHPT, VDI, and stage 3 or 4 CKD were stratified according to stage and received daily extended-release bone Ethylene glycol (ERC) or placebo treatment. After 26 weeks of treatment, all subjects were sorted by serum total 25-hydroxyvitamin D levels and divided into quintiles to examine the degree of vitamin D supplementation in relation to plasma iPTH, serum bone turnover markers, calcium, phosphorus, and complete composition. Relationship of changes in fibroblast growth factor 23 (FGF23) and vitamin D metabolites, estimated glomerular filtration rate (eGFR), and urinary calcium to creatinine (Ca:Cr) ratio.
[0101] Specifically, two identical 26-week multicenter randomized, double-blind, placebo-controlled design studies recruited a total of SHPT (plasma iPTH ≥85 and 2 ) and VDI (serum total 25-hydroxyvitamin D ≥ 10 and 0.2, nephrotic range proteinuria (>3 mg / mg Cr), and history of...
Embodiment 2
[0131] This example describes a structured chart review of patients with stage 3 or 4 chronic kidney disease with vitamin D insufficiency and secondary hyperparathyroidism who were being treated with extended-release calciferidiol or other relevant comparators. This study addresses mineral and bone disease before dialysis: a real-world assessment of the risks and effectiveness of the current SHPT treatment approach (MBD-AWARE).
[0132] Target
[0133] The overall goal of this study was to generate preliminary real-world evidence that: (a) extended-release calcifediol (ERC) is used in the treatment of adult patients with stage 3 or 4 chronic kidney disease (CKD) and vitamin D insufficiency (VDI) secondary to Safety and Efficacy in Sexual Hyperparathyroidism (SHPT): and (b) Utilization, safety, and efficacy of other vitamin D therapies (OVDT) are considered standard of care for the treatment of SHPT in these patients. OVDTs include nutritional vitamin D (NVD), defined as orall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com