Parathyroid hormone variants
A parathyroid hormone and variant technology, applied in the direction of parathyroid hormone, hormone peptides, antibody mimics/stents, etc., can solve problems such as poor control, short half-life, daytime serum calcium fluctuations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0210] The following examples illustrate certain aspects of the specification. These examples should not be construed as limiting, as these examples merely provide a specific understanding and practice of the embodiments and their various aspects.
example 1
[0211] Example 1: Production and purification of Fc fusion PTH variants (PTH-66 as an example)
[0212] PTH-66 was transiently expressed in CHO cells and the conditioned medium was stored at -20°C until further use. Thaw conditioned media in a 25 °C water bath and filter through a 0.2 µm bottle top filter unit. PTH-66 protein was purified on protein A derivatized resin MabSelect SuRe following the manufacturer's protocol (Figure 8). MabSelect SuRe columns were pre-equilibrated with PBS. Thawed conditioned medium was loaded onto the column and washed extensively with PBS. PTH-66 protein was eluted by step elution with 100 mM sodium citrate buffer, pH 3.2. Eluted fractions were analyzed on SDS-PAGE (Figure 9). The pH of the pooled eluted fractions was raised to about pH 6 with 1M Tris pH 10. The neutralized pooled elution fractions were dialyzed into PTH-66 storage buffer 20 mM sodium phosphate buffer, 50 mM sodium chloride, 26 mg / ml mannitol, pH 6.0, and stored at -80°C. ...
example 2
[0216] Example 2: Effect of C-terminal truncation of PTH-Fc fusions
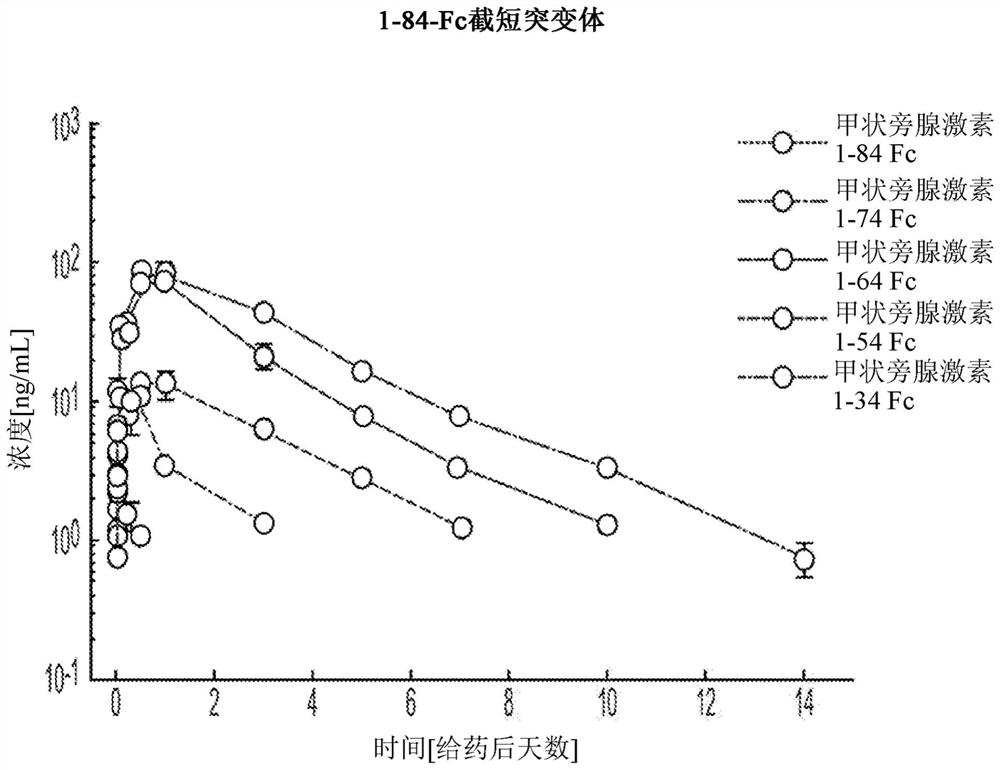
[0217] PTH-Fc fusion variants comprising truncated PTHs of different lengths were prepared according to the method outlined in Example 1. The following PTH-Fc fusion variants were generated: PTH-66 (PTH(1-74)-Fc), PTH-67 (PTH(1-64)-Fc), PTH-68 (PTH(1-54)-Fc ), PTH-69 (PTH(1-44)-Fc) and PTH(1-34)-Fc.
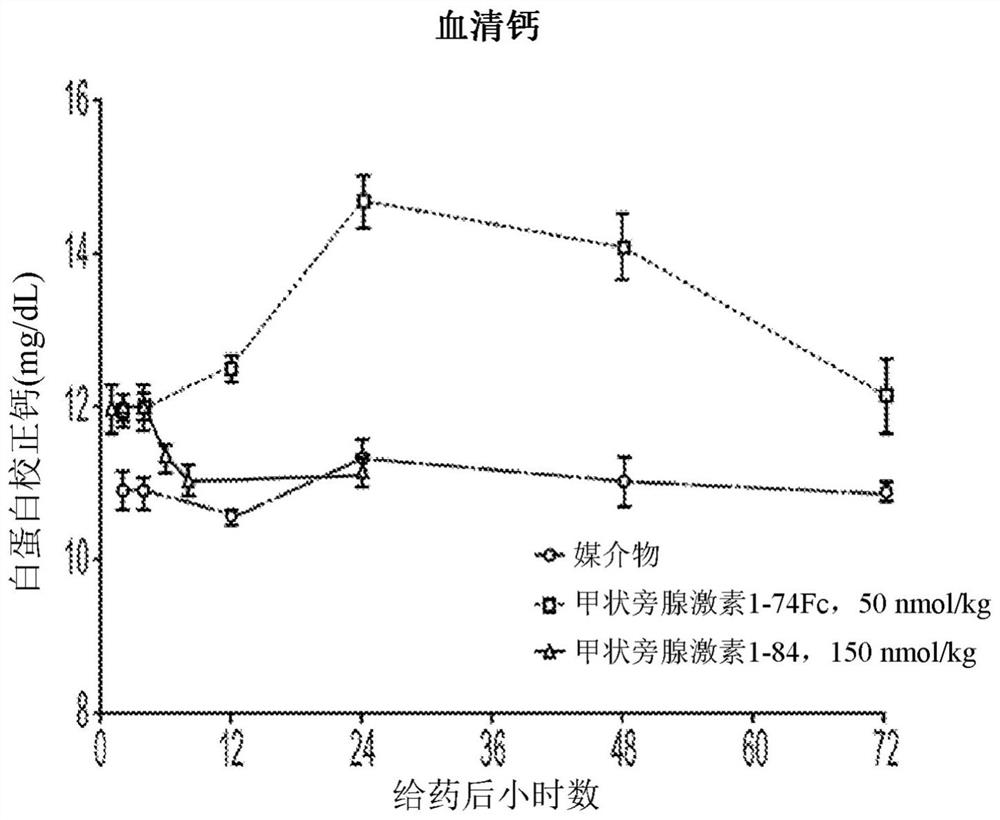
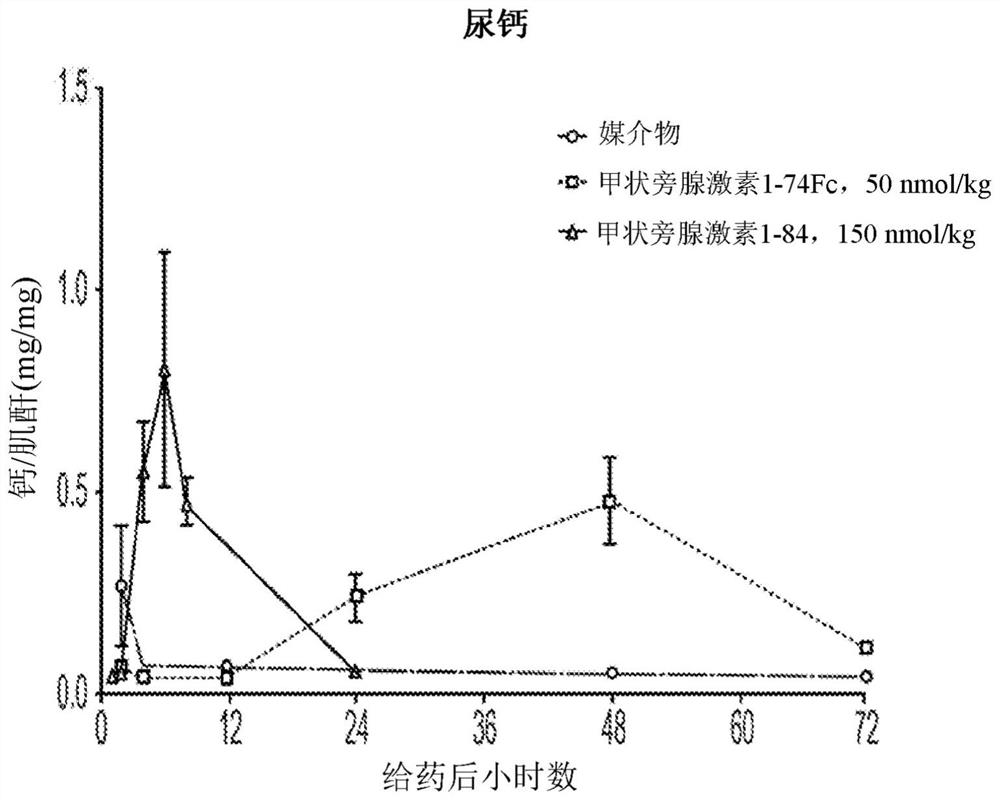
[0218] After a single subcutaneous treatment of Spraque-Dawley rats with truncated variants and full-length PTH(1-84)-Fc at 0.25 mg / kg was assessed using the Immunotopics Bioactivity ELISA Kit (60-3000). Serum concentration of PTH, and results in figure 1 shown in .
[0219] Such as figure 1 As shown, C-terminal truncation of PTH surprisingly and unexpectedly improves the exposure and pharmacokinetics of PTH-Fc fusions.
[0220] As shown, the truncated PTH-Fc fusion variants of the present invention are superior to native length PTH(1-84)-Fc fusions and teriparatide-Fc (PTH(1-34)-Fc fusions) . Even mor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com