Nicotinamide for treating dyslipidemia
A technology for dyslipidemia and nicotinamide, which is applied in the direction of medical preparations containing active ingredients, metabolic diseases, drug combinations, etc., and can solve problems such as limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
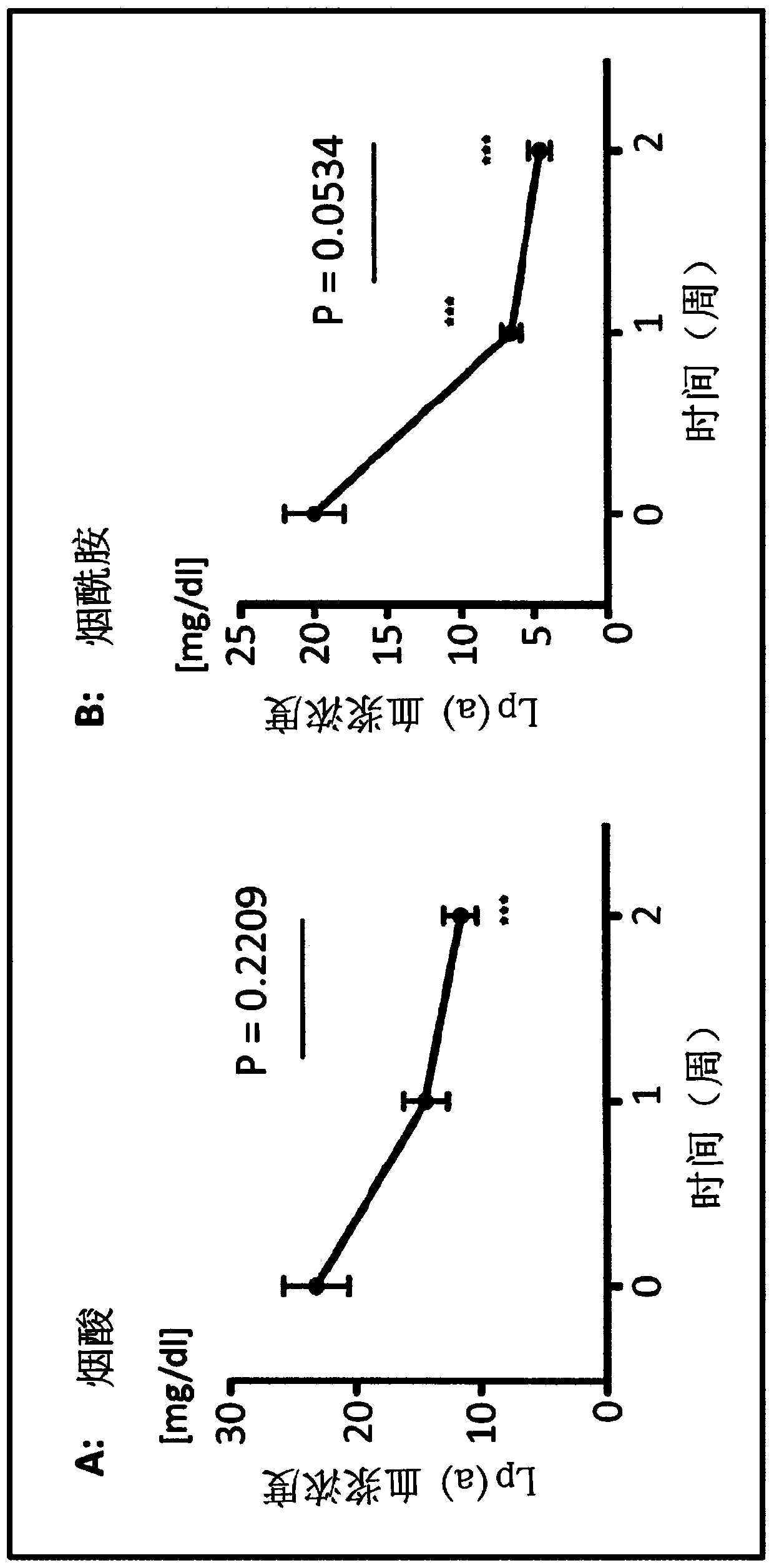
[0157] Niacinamide improves Lp(a) levels in CKD patients.
[0158] To investigate the possible effect of nicotinamide on lowering serum Lp(a) levels, the inventors conducted preclinical studies in a transgenic mouse model. The biosynthesis of Lp(a) exists only in humans and Old World monkeys, which complicates the direct study of Lp(a) metabolism using animal models (Kostner, 2013(72)). Lp(a) consists of LDL covalently bound to Apo(a). Plasma Lp(a) levels in humans are highly correlated with Apo(a) synthesis. Since Lp(a) synthesis is limited to primates, a transgenic mouse model was adopted in which the entire Apo(a) gene including the promoter region was introduced into the mouse genome (Chennamsetty, 2012(31)). In transgenic Apo(a) mice (tg-Apo(a)), Apo(a) is mainly expressed in female mice. After these female mice were orally treated with food containing 1% niacin, plasma Apo(a) protein was reduced by 43%, and Apo(a) mRNA transcription in hepatocytes was reduced by 65% ...
Embodiment 2
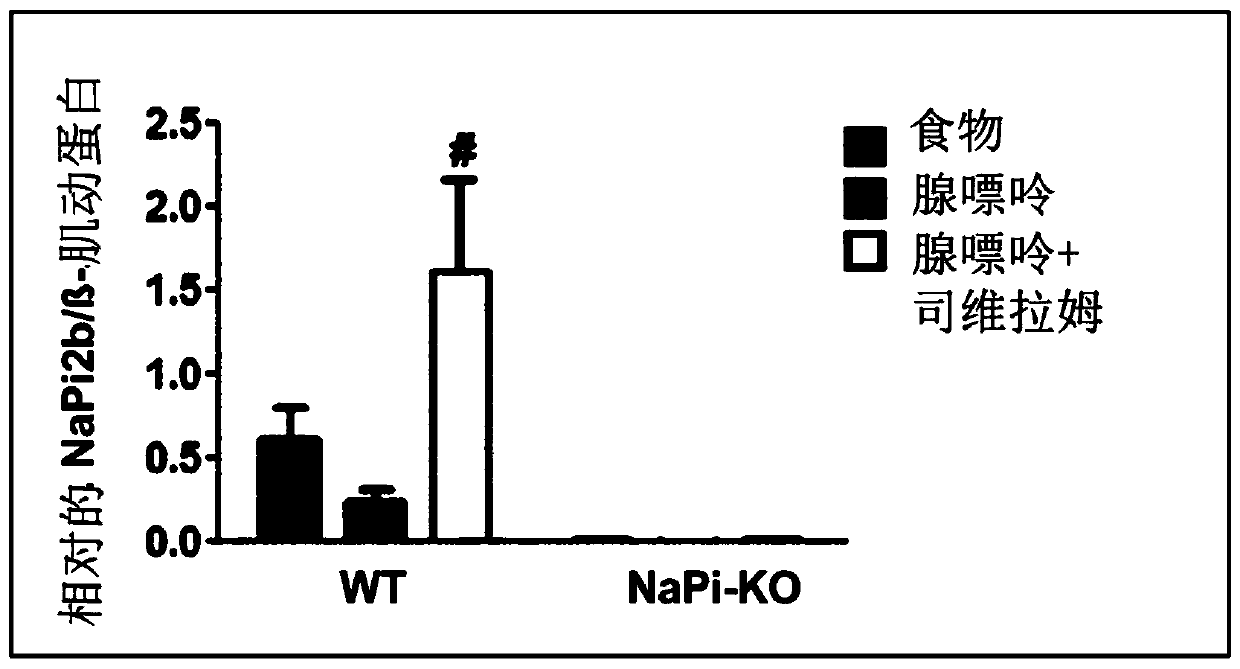
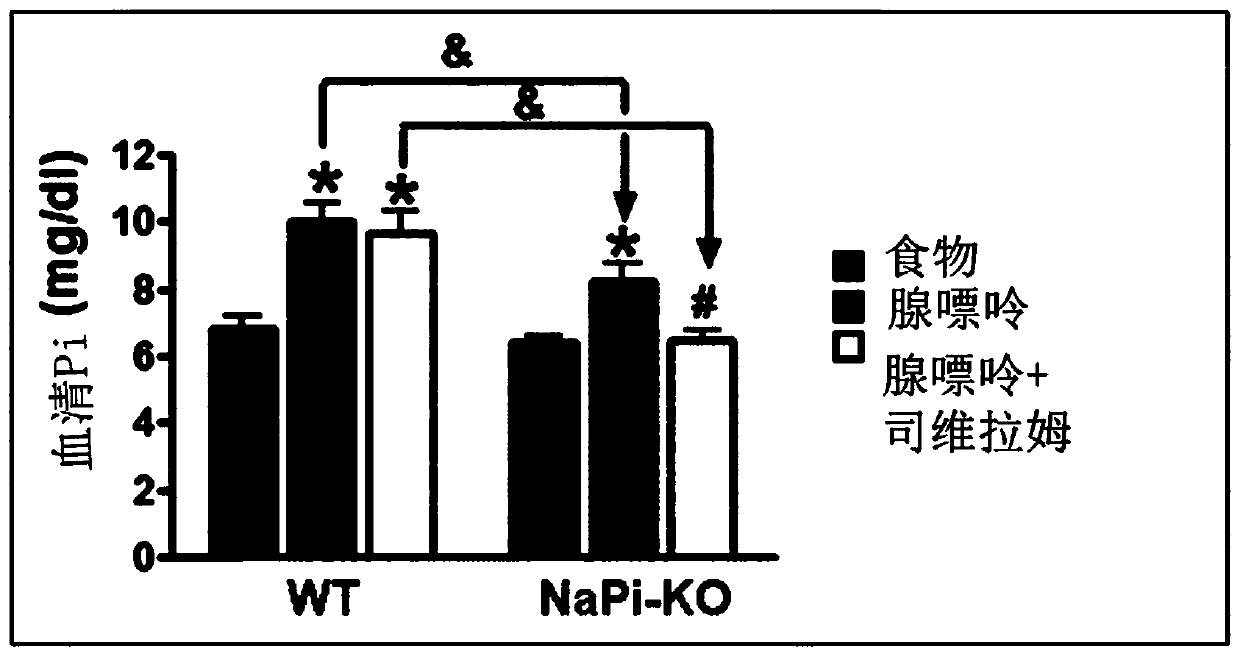
[0164] The combined therapeutic approach of nicotinamide and phosphate binders is particularly promising in the treatment of hyperphosphatemia in particular, because the pharmacological target of nicotinamide, the cotransporter NaPi2b, is strongly upregulated under treatment with phosphate binders . In this regard, it should also be noted again that phosphate binders, such as sevelamer and / or its derivatives, can negatively affect nicotinamide intake, which can be mitigated when co-administered with modified-release nicotinamide. Negative effects of phosphate binders.
[0165] There are two different mechanisms to explain intestinal phosphate absorption. Under conditions of normal dietary phosphate supply, passive paracellular diffusion accounts for the majority of phosphate uptake. In cases of dietary phosphate restriction, or treatment with phosphate binders to prevent intestinal phosphate absorption, the active cotransporter-driven process of absorption is enhanced, leadi...
Embodiment 3
[0182] Furthermore, most phosphate binders do not specifically react with phosphate, but also bind to other small polar molecules in the gut, potentially inhibiting their intestinal absorption (see e.g. Neradova, 2016(55)) . Indeed, when nicotinamide is combined with the phosphate binder sevelamer, with its known potential for broad gut interactions, nicotinamide was ineffective for the treatment of hyperphosphatemia ( See Olivero, 2006(43)). Sevelamer Carbonate The summary statement of the product characteristics of the drug is as follows: "Sevelamer is not absorbed and may affect the bioavailability of other drugs. When administered with any drug, if the decrease in bioavailability can have adverse effects on safety or efficacy." clinically significant effect, the drug should be administered at least 1 hour before or 3 hours after taking sevelamer, or physicians should consider monitoring blood levels." Due to the mode of action of phosphate binders, daily It must be tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com