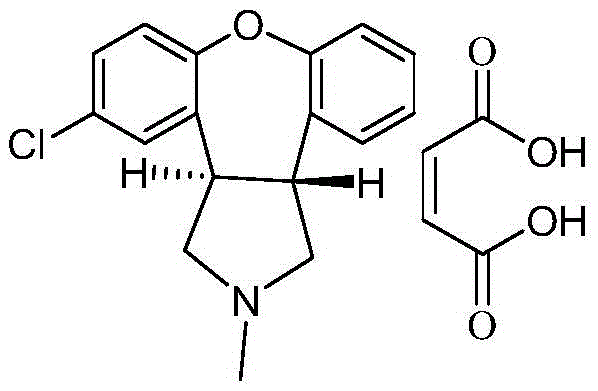
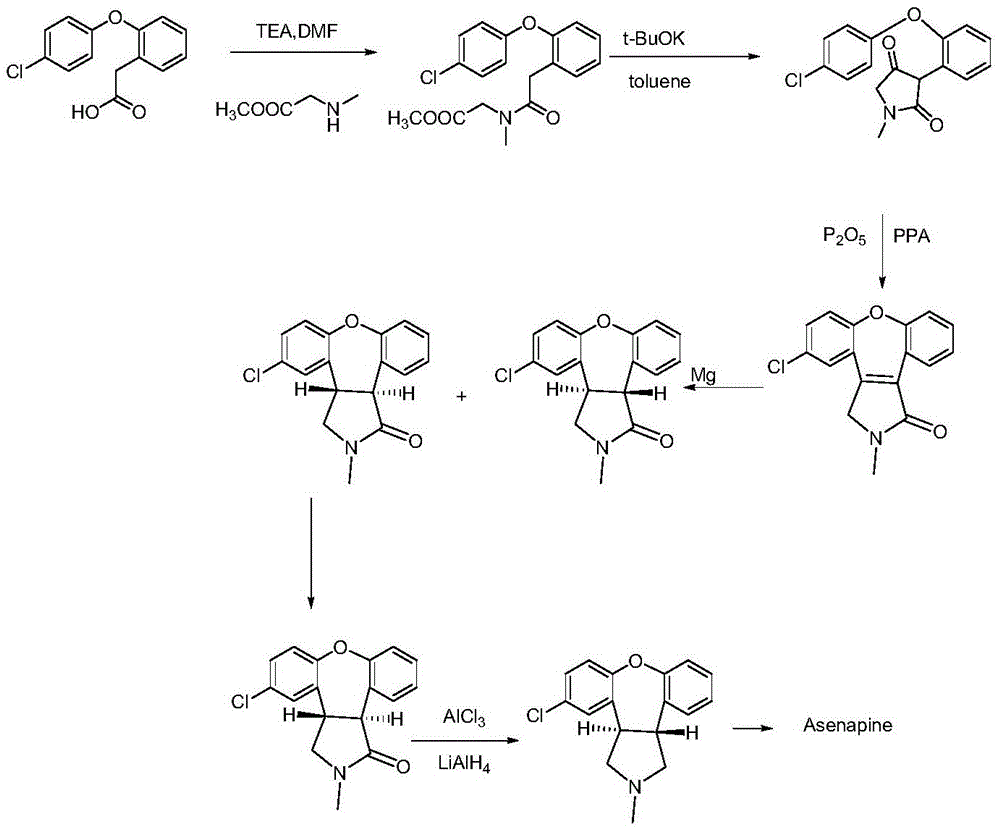
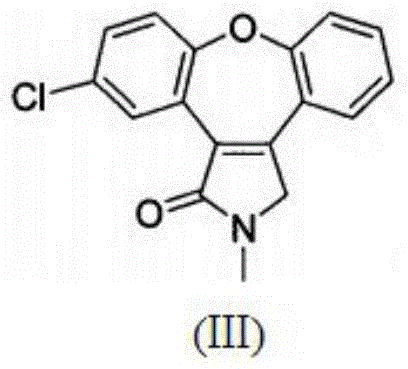
A kind of preparation method of asenapine key intermediate
An intermediate and key technology, applied in the field of chemical synthesis, can solve problems such as high reaction temperature, cumbersome operation, environmental pollution, etc., and achieve the effects of simplified reaction steps, simple operation, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 30ml of dichloromethane to a 100ml three-necked flask, add 5.6g of starting material 2-(4-chlorophenoxy)phenylacetic acid, cool down to 0-5°C, add dropwise pivaloyl chloride / dichloromethane=12.1g / 10ml, add 5.6g of sarcosine methyl ester hydrochloride to another 250ml three-necked flask, cool down to below 10°C, add 4.1g of triethylamine and stir for 30 minutes, slowly add the dichloromethane solution of mixed anhydride (5.6g / 20ml ), controlled dropwise under 15°C; after adding, keep stirring for 3 to 4 hours, add 50ml of purified water and stir for 5 minutes, extract and separate the liquid, extract the organic phase with 50ml of purified water again, concentrate the organic phase to constant weight, and obtain brown Or tan oily substance, that is, 6.2 g of the intermediate compound of formula (I), with a yield of 83.8%.
Embodiment 2
[0036] Add 30ml of dichloromethane to a 100ml three-necked flask, add 5.6g of 2-(4-chlorophenoxy)phenylacetic acid, cool down to -30~5°C, add methanesulfonyl chloride / dichloromethane=2.3g / 10ml dropwise, Add 2.8g of sarcosine methyl ester hydrochloride to another 250ml three-necked flask, cool down to below 10°C, add 2.0g of triethylamine and stir for 30 minutes, slowly add the dichloromethane solution of mixed anhydride (5.6g / 20ml) dropwise, Add dropwise at a temperature below 15°C; after adding, keep stirring for 1 to 3 hours, add 50ml of purified water and stir for 5 minutes, extract and separate the liquid, extract the organic phase with 50ml of purified water again, concentrate the organic phase to constant weight, and obtain brown or brown Brown oily substance, 6.1 g of intermediate compound of formula (I), yield 82.4%.
Embodiment 3
[0038] Add 25ml of toluene and 1.2g of potassium tert-butoxide to a 50ml three-necked flask, add the toluene solution (3.5g / 25ml) of the intermediate of formula (I) prepared in the above example below 20°C, stir overnight at room temperature, add 75ml of purified Water extraction, the water phase was extracted three times with 25ml EA, the organic phase was combined, extracted once more with 25ml purified water, and the water phase was combined; the water phase was adjusted to pH=1.0 with 1N hydrochloric acid (turbidity appeared), crystal growth was stirred for 3 to 4 hours, and filtered , the filter cake was rinsed once with 20 ml of purified water, and the solid was dried under reduced pressure at 40° C. for 5 to 6 hours to obtain 2.3 g of the intermediate compound of formula (II), with a yield of 71.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com