Preparation method of asenapine intermediate
A technology for intermediates and compounds, applied in the field of organic synthesis, can solve the problems of a lot of waste water, low reaction yield, difficult removal of impurities, etc., and achieve the effects of reasonable process conditions, high reaction yield and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
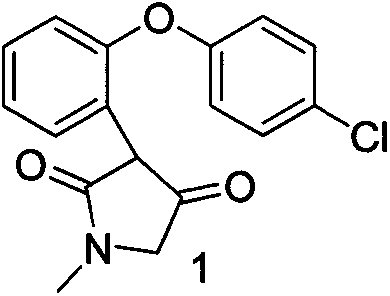
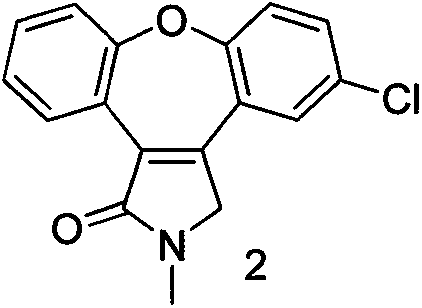
[0022] Example 15-chloro-2,3-dihydro-2-methyl-1H-dibenzo[2,3:6,7]oxepin[4,5-c]pyrrol-1-one (compound 2) Preparation
[0023] Add 232 g of 3-(2-(4-chlorophenoxy)-phenyl)-4-hydroxy-1-methyl-1H-pyrrol-2(5H)-one (compound 1) into a 5-liter four-necked reaction flask , add 2 liters of trifluoromethanesulfonic acid at one time, and raise the temperature to 110°C for 10-15h.
[0024] After the reaction, most of the trifluoromethanesulfonic acid was distilled off under reduced pressure, the residue was cooled to room temperature, poured into ice water, a large amount of solids precipitated, and filtered. The solid was recrystallized with acetone and water to obtain 135 g of the product with a yield of 71% and a purity of >96%.
[0025] HNMR data (DMSO-d6 solvent)
[0026] 3.10(d, J=2.5Hz), 4.70(d, 2H, J=4.2Hz), 7.2-7.6(m, 6H), 8.1(s, 1H)
Embodiment 211
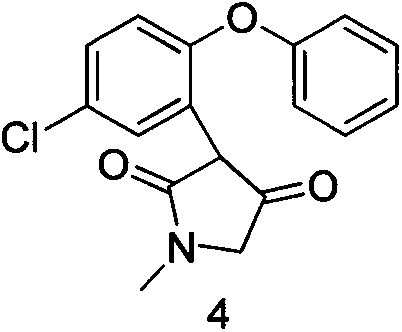
[0027] Example 211-chloro-2,3-dihydro-2-methyl-1H-dibenzo[2,3:6,7]oxepin[4,5-c]pyrrol-1-one (compound 5) Preparation
[0028] Add 232 g of 3-(5-chloro-2-phenoxyphenyl)-1-methyl-1H-pyrrol-2(4H)-one (compound 4) into a 5-liter four-necked reaction flask, and add 2 liters at a time Trifluoromethanesulfonic acid, heated to 110 ° C for 10-15h. After the reaction, most of the trifluoromethanesulfonic acid was distilled off under reduced pressure, the residue was cooled to room temperature, poured into ice water, a large amount of solids precipitated, and filtered. The solid was recrystallized with acetone and water to obtain 145 g of the product with a yield of 76% and a purity of >95%.
Embodiment 3
[0029] The preparation of embodiment 3 asenapine
[0030] With compound 2 or 5, high-purity asenapine (trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-diphenyl and[2,3:6,7]oxazolo[4,5-c]pyrrole).
[0031] Comparative example 15-chloro-2,3-dihydro-2-methyl-1H-dibenzo[2,3:6,7]oxepin[4,5-c]pyrrol-1-one (compound 2) Preparation (polyphosphoric acid method)
[0032] Add 232 g of 3-(5-chloro-2-phenoxyphenyl)-1-methyl-1H-pyrrol-2(4H)-one (compound 1) into a 5-liter four-necked reaction flask, and add 1 L of polyphosphoric acid , heated to 150 ° C for 15 h. The reaction is still not over, add phosphorus pentoxide, heat up to 160 °C, after the reaction, cool the solution to 50 °C, pour it into ice water, a lot of solids precipitate out, filter it. The solid was recrystallized with acetone and water to obtain 110 g of the product with a yield of 50% and a purity of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com