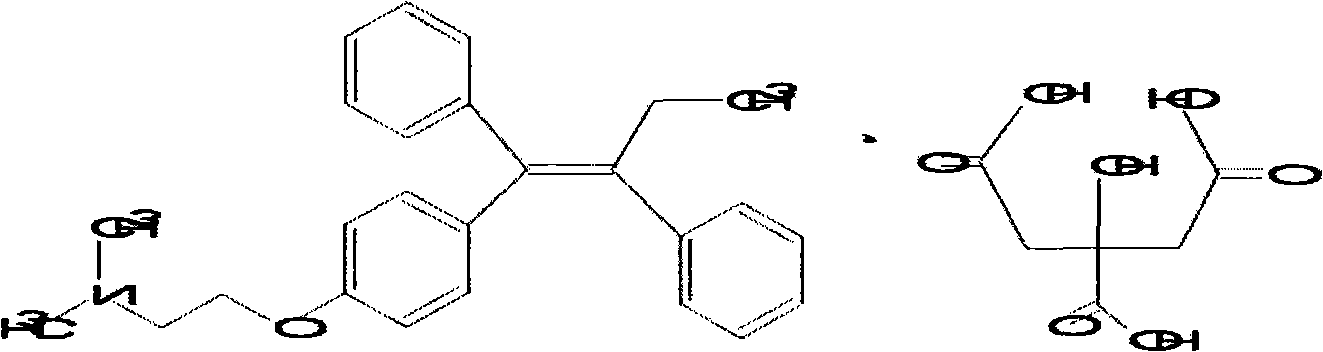
Acidum citricum nolvadex capsule and preparation method thereof
A technology of tamoxifen and citric acid, applied in the field of medicine, can solve the problems of slow dissolution, poor stability, low drug absorption and bioavailability, etc., and achieves the advantages of simple preparation, stable preparation, prevention and slowing down of drug decomposition. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Weigh an appropriate amount of povidone K30 and add ethanol to prepare a solution with a concentration of 10% by weight, and set aside.
[0023] 2. Crush tamoxifen citrate and pass through a 100-mesh sieve, and microcrystalline cellulose, crospovidone, starch, and magnesium stearate are respectively passed through a 100-mesh sieve for later use.
[0024] 3. Weigh 15.17g of tamoxifen citrate, 70g of starch, 60g of microcrystalline cellulose, and 5g of crospovidone, put them in a wet granulator and mix them well, add an appropriate amount of binder, and stir to make a soft material. Wet granules were made through a 20-mesh sieve.
[0025] 4. Dry the wet granules in an oven at 50-60°C for 1-2 hours.
[0026] 5. Sorting the dry granules through a 14-mesh sieve.
[0027] 6. Transfer the dry granules to a V-shaped blender, add 0.75g of magnesium stearate and mix well.
[0028] 7. After measuring the percentage of tamoxifen citrate in the granules, fill the capsules and ...
Embodiment 2
[0030] The implementation is basically the same as in Example 1, the difference is that
[0031] The weight of each component is 30.34 g of tamoxifen citrate, 75 g of starch, 90 g of microcrystalline cellulose, 5 g of crospovidone, and 1 g of magnesium stearate.
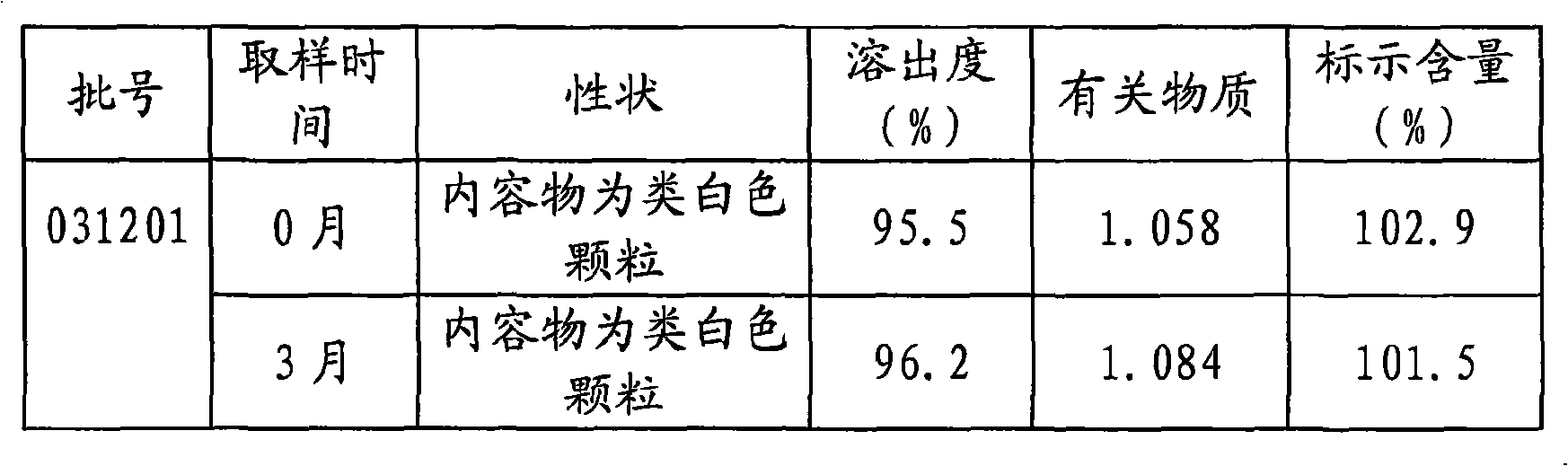
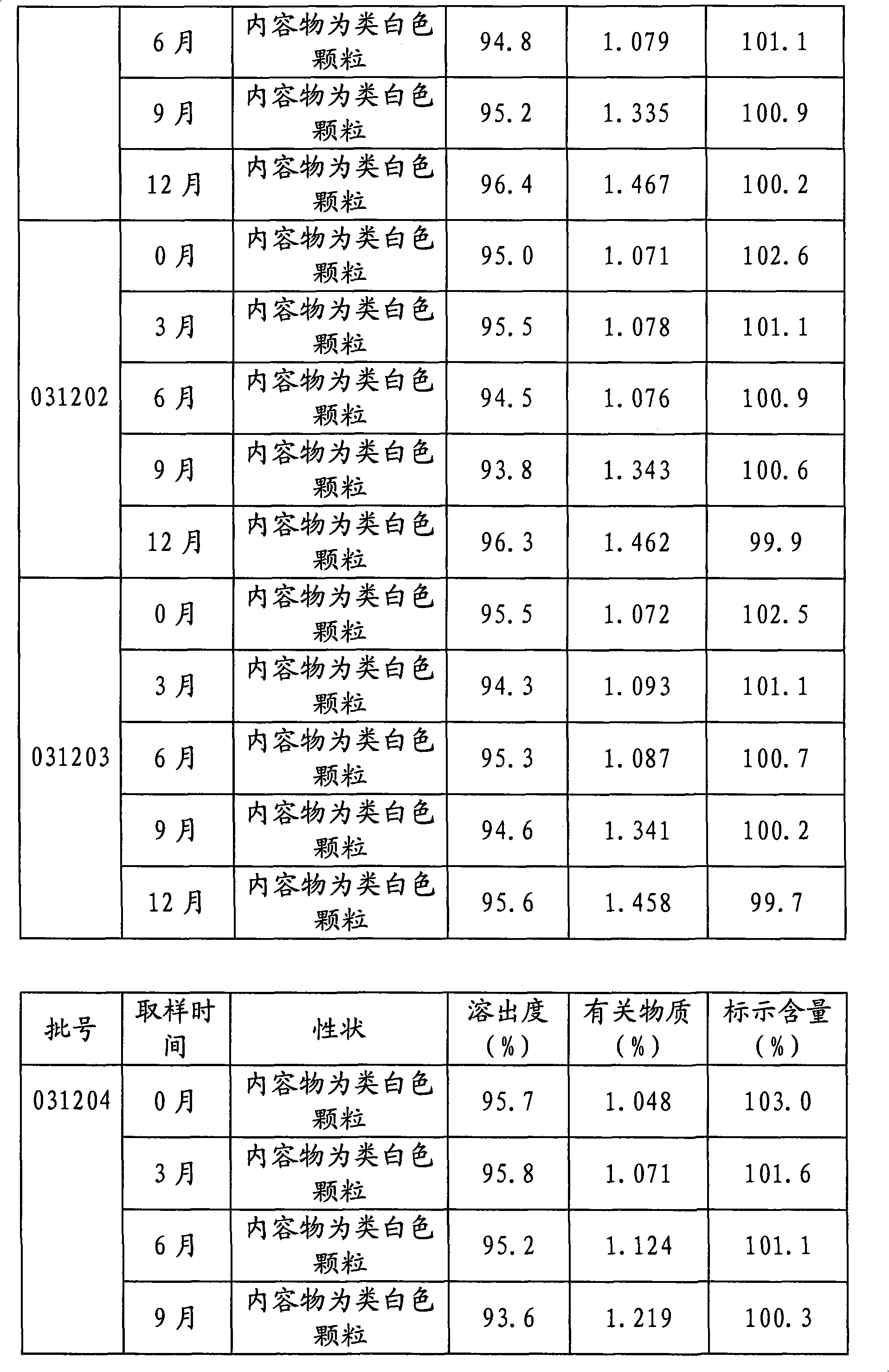
[0032] The following two tables are the long-term stability test data of tamoxifen citrate capsules prepared according to Examples 1 and 2 respectively.
[0033]
[0034]
[0035]
[0036] The test data shows that the tamoxifen citrate capsules prepared according to the content of the present invention have no significant change in each main index after long-term storage, and the product has good stability and safety, high dissolution rate, and guaranteed curative effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com