Preparation method of asenapine intermediate
A technology of intermediates and compounds, which is applied in the field of organic synthesis, can solve the problems of low reaction yield, excessive waste water, difficult to remove impurities, etc., and achieve the effects of high reaction yield, reasonable process conditions and low production cost
Active Publication Date: 2010-10-06
SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
View PDF6 Cites 15 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
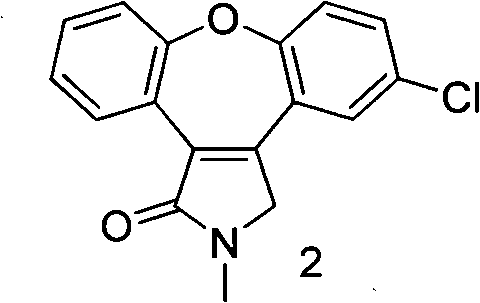
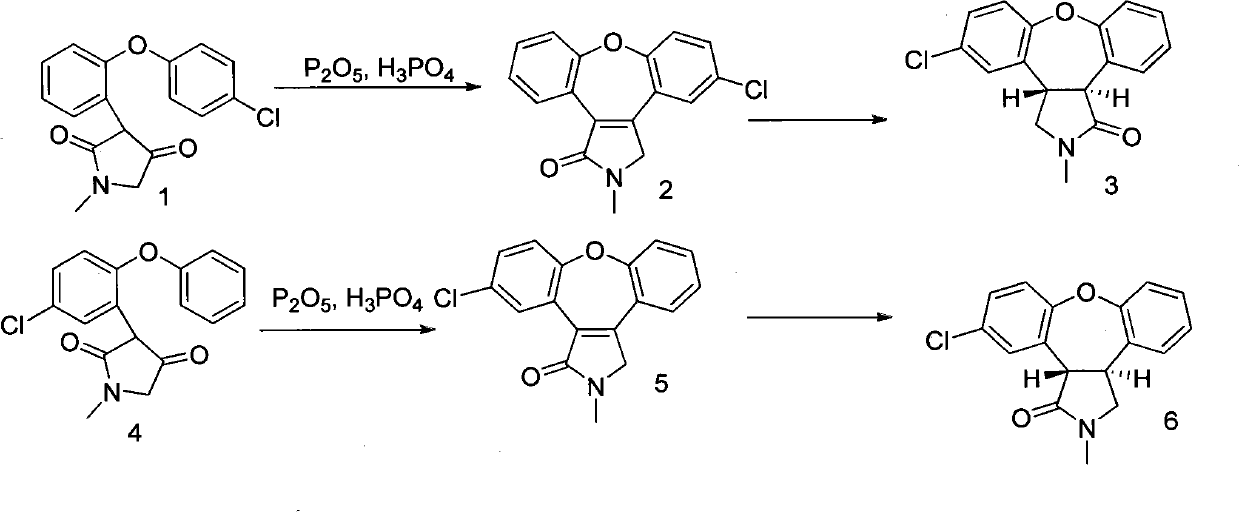
When the final product asenapine (compound 7) is synthesized by the above-mentioned route, there are problems that the reaction yield of compound 2 or 5 is low, impurities are difficult to remove, and there is a lot of waste water
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 15
Embodiment 211
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
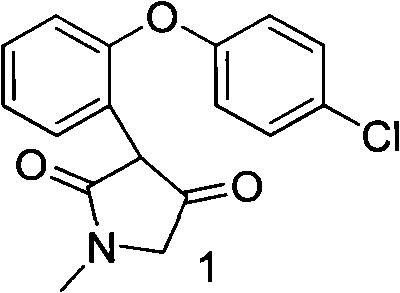
The invention discloses a novel preparation method of an asenapine intermediate, comprising the following steps of: carrying out cyclization in protonic acid including trifloromethanesulfonic acid by taking 3-(2-(4- chlorophenoxyl)-phenyl-4-hydroxyl-1-methyl-1H-pyrrole-2(5H)-ketone (compound 1) or 3-(5-chlorine-2-phenoxyl phenyl)-1-methyl-1H-pyrrole-2(4H)-ketone (compound 4) as a raw material to generate key intermediate compounds 2 and 4 of asenapine; and carrying out reduction reaction to obtain asenapine. The invention has the advantages of novel process route, high reaction yield and low production cost, and has greater application value and social economic effect.
Description
technical field The invention relates to a chemical synthesis method, in particular to a novel preparation method of an intermediate of Asenapine which can be used as a schizophrenic, and belongs to the field of organic synthesis. technical background The trade name of Asenapine is Saphris, developed by Organon BioSciences and produced by ScheringPlough. On August 14, 2009, the FDA approved the drug for the emergency treatment of adults with schizophrenia, mania, or mixed episodes with type I bipolar disorder. English chemical name of asenapine: (3aR, 12bR)-rel-5-chloro-2, 3, 3a, 12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4 ,5-c]pyrrole; Chinese chemical name: trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxa Zhuo[4,5-c]pyrrole; molecular formula: C17H16ClNO; relative molecular mass: 285.77; CAS registration number: 65576-45-6. Preparation. WO2006106136, WO2007046554, US20090209608, and WO2008078482 reported that starting materials 1 and 5 were...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D491/044
Inventor 高强薛吉军曾亮彭小波郑保富
Owner SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com