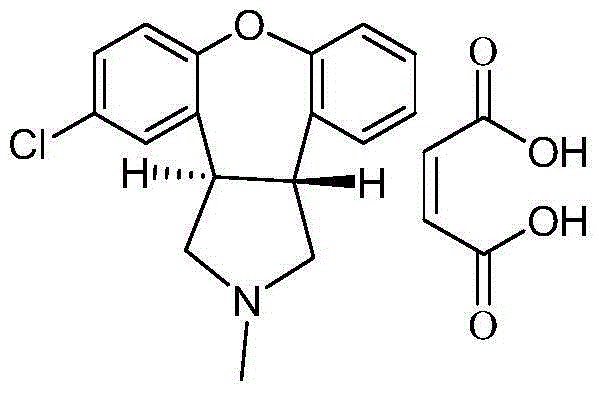
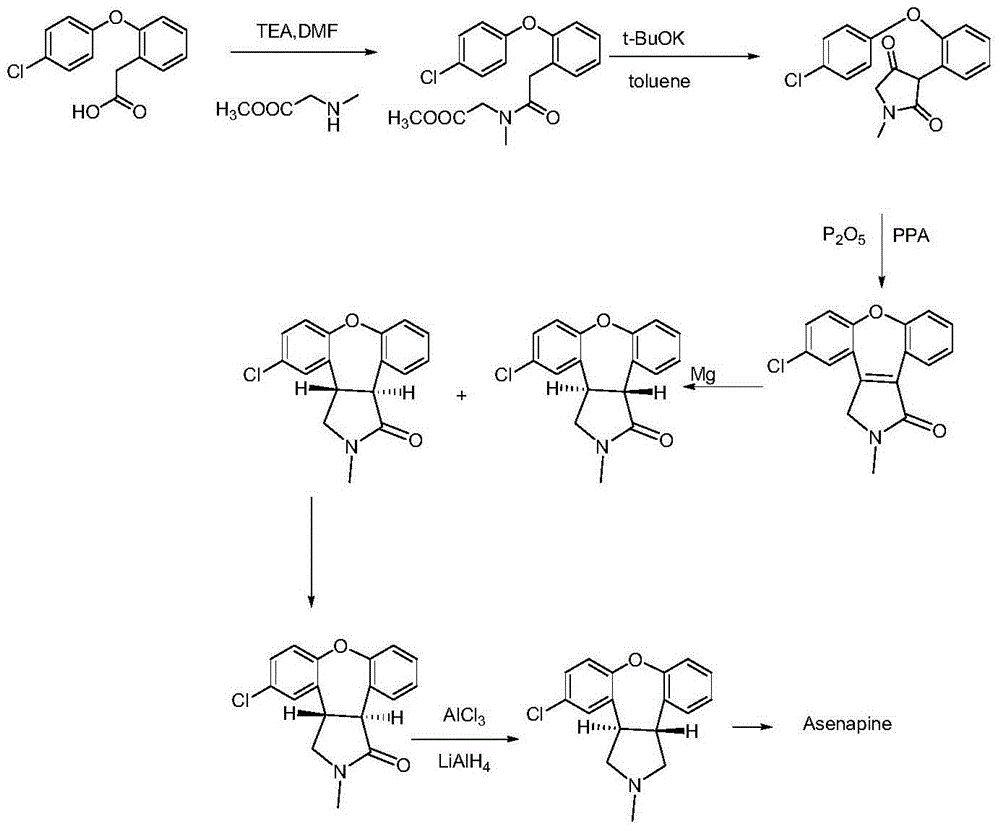
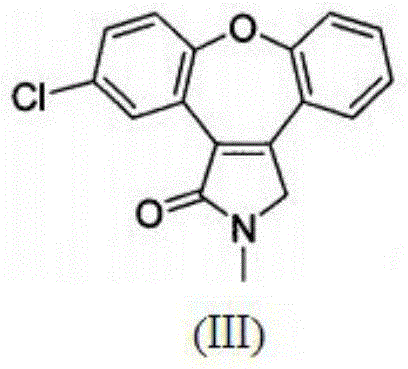
Preparation method of asenapine key intermediate
An intermediate and key technology, applied in the field of chemical synthesis, can solve the problems of high reaction temperature, complicated operation, low yield and the like, and achieve the effects of simplified reaction steps, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 30ml of dichloromethane into a 100ml there-necked flask, add 5.6g of starting material 2-(4-chlorophenoxy)phenylacetic acid, cool to 0~5°C, dropwise add pivaloyl chloride / dichloromethane=12.1g / 10ml, add 5.6g of sarcosine methyl ester hydrochloride to another 250ml three-necked bottle, cool down to below 10°C, add 4.1g of triethylamine and stir for 30 minutes, slowly add the dichloromethane solution of mixed anhydride (5.6g / 20ml) dropwise. ), and added dropwise under the control of 15°C; after adding, keep stirring for 3-4 hours, add 50ml purified water and stir for 5 minutes, extract and separate the liquid, extract the organic phase again with 50ml purified water, and concentrate the organic phase to constant weight to obtain a brown color or tan oily substance, namely the intermediate compound of formula (I) 6.2g, the yield is 83.8%.
Embodiment 2
[0036] Add 30ml of dichloromethane into a 100ml three-necked flask, add 5.6g of 2-(4-chlorophenoxy)phenylacetic acid, cool down to -30~5°C, dropwise add methanesulfonyl chloride / dichloromethane=2.3g / 10ml, in In addition, add 2.8g of sarcosine methyl ester hydrochloride to a 250ml three-necked flask, cool the temperature to below 10°C, add 2.0g of triethylamine and stir for 30 minutes, and slowly add the dichloromethane solution of mixed anhydride (5.6g / 20ml) dropwise, Control the dropwise addition below 15°C; after the addition, maintain stirring for 1 to 3 hours, add 50 ml of purified water and stir for 5 minutes, extract and separate, extract the organic phase again with 50 ml of purified water, and concentrate the organic phase to constant weight to obtain brown or brown. The brown oil, namely the intermediate compound of formula (I), was 6.1 g, and the yield was 82.4%.
Embodiment 3
[0038] Add 25ml of toluene and 1.2g of potassium tert-butoxide into a 50ml three-necked flask, add the toluene solution (3.5g / 25ml) of the intermediate of formula (I) prepared in the above example below 20°C, stir at room temperature overnight, add 75ml of purification Water extraction, the water phase was extracted three times with 25ml EA, the organic phase was combined, extracted once again with 25ml purified water, and the water phase was combined; the water phase was adjusted to pH=1.0 with 1N hydrochloric acid (turbidity), and the crystal was stirred for 3 to 4 hours, and filtered. , the filter cake was rinsed once with 20 ml of purified water, and the solid was dried under reduced pressure at 40° C. for 5 to 6 hours to obtain 2.3 g of the intermediate compound of formula (II) with a yield of 71.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com