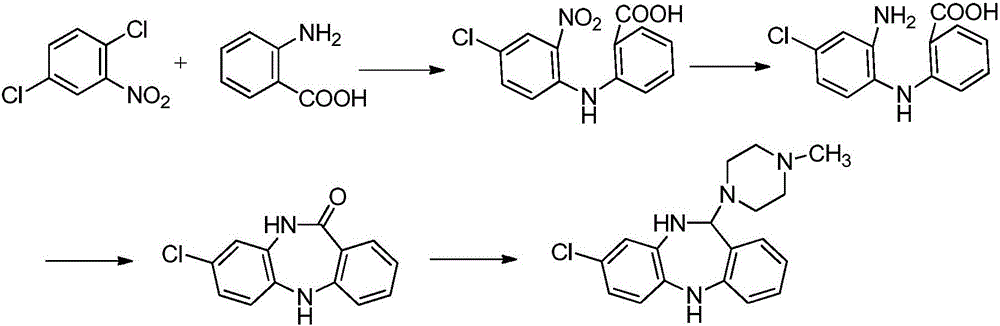
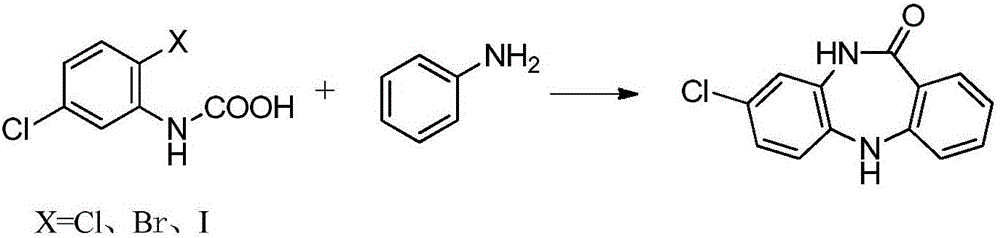
Key clozapine intermediate synthesis method
A synthesis method and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of many types of raw materials, low yield, complicated reaction steps, etc., and achieve the effects of simple steps, avoiding environmental pollution, and avoiding cost increase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Under argon protection, add 20.5 g of N-(2-chloro-5-chlorophenyl) carbamic acid, 82 mL of aniline, 13.8 g of potassium carbonate and 0.02 g of cuprous chloride to the four-necked flask in sequence, raise the temperature to 153°C, and stir React for 2-3 hours. TLC followed the reaction, the reaction was completed, cooled slightly, filtered, the filtrate was distilled under reduced pressure, and the excess aniline solvent was evaporated, and the remaining solid was washed with water three times and dried to obtain 8-chloro-5,10-dihydro-11H-dibenzo[ b,e][1,4]-Diazepam -11-one 21.2g, yield 86.8%.
example 2
[0024] Under argon protection, add 20.5g of N-(2-chloro-5-chlorophenyl)carbamate, 102.5mL of aniline, 12.7g of sodium carbonate and 0.13g of cuprous bromide to the four-necked flask in sequence, and heat up to 153°C , stirring the reaction for 2-3 hours. TLC followed the reaction, the reaction was completed, cooled slightly, filtered, the filtrate was distilled under reduced pressure, and the excess aniline solvent was evaporated, and the remaining solid was washed with water three times and dried to obtain 8-chloro-5,10-dihydro-11H-dibenzo[ b,e][1,4]-Diazepam -11-one 21.7g, yield 88.9%.
example 3
[0026] Under argon protection, add 25.0 g of N-(2-bromo-5-chlorophenyl) carbamic acid, 150 mL of aniline, 7.4 g of lithium carbonate and 0.02 g of cuprous chloride to the four-necked flask in sequence, raise the temperature to 153°C, and stir React for 2-3 hours. TLC followed the reaction, the reaction was completed, cooled slightly, filtered, the filtrate was distilled under reduced pressure, and the excess aniline solvent was evaporated, and the remaining solid was washed with water three times and dried to obtain 8-chloro-5,10-dihydro-11H-dibenzo[ b,e][1,4]-Diazepam -11-one 21.8g, yield 89.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com