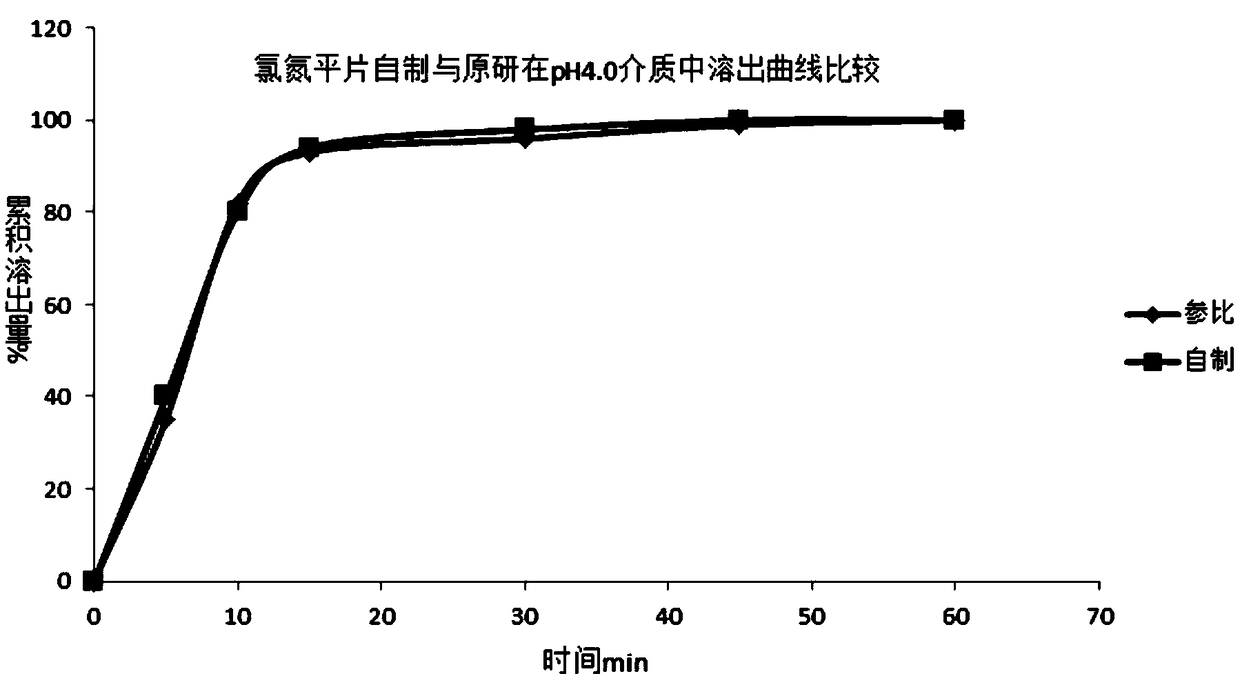
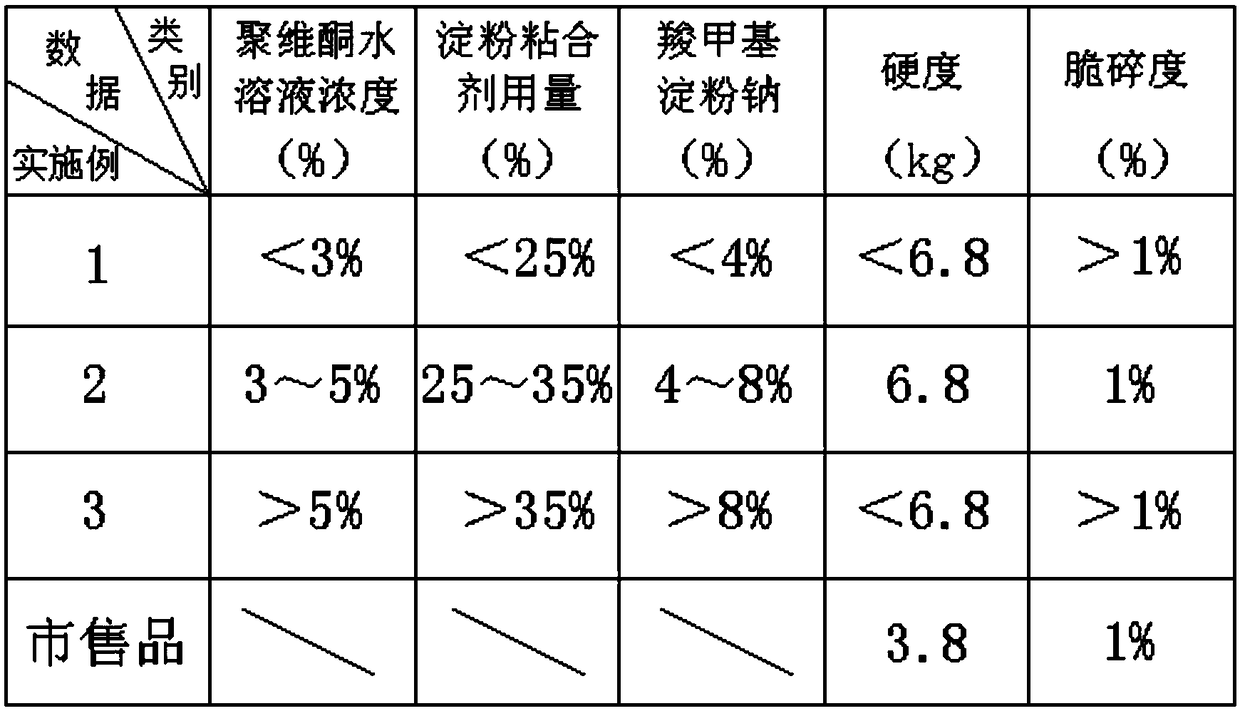
Clozapine tablet and preparation method thereof
A clozapine tablet and clozapine technology are applied in the field of clozapine tablet and its preparation, which can solve the problems of poor tablet core hardness, poor compressibility, splintering, etc. Safe, high similarity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The invention provides a clozapine tablet and a preparation method thereof. The main ingredients and mass parts of the clozapine tablet are: 25 parts of clozapine, 61.2 parts of microcrystalline cellulose, 3.8 parts of lactose, povidone Appropriate amount, 0.5 parts of magnesium stearate, 1.4 parts of talcum powder, 0.4 parts of silicon dioxide;
[0026] Wherein, the preparation method of described clozapine tablet comprises the following steps:
[0027] Step 1: passing clozapine through a 100-mesh sieve for subsequent use;
[0028] Step 2: take by weighing clozapine, microcrystalline cellulose, lactose by the prescription amount in claim 1 and place in the wet mixing granulator together and mix evenly;
[0029] Step 3: Weigh an appropriate amount of povidone, dissolve it in water and prepare an aqueous solution with a concentration of less than 3%;
[0030] Step 4: Add an appropriate amount of starch binder to the mixed powder in the previous step to make a suitable ...
Embodiment 2
[0035] The invention provides a clozapine tablet and a preparation method thereof. The main ingredients and mass parts of the clozapine tablet are: 25 parts of clozapine, 61.2 parts of microcrystalline cellulose, 3.8 parts of lactose, povidone Appropriate amount, 0.5 parts of magnesium stearate, 1.4 parts of talcum powder, 0.4 parts of silicon dioxide;
[0036] Wherein, the preparation method of described clozapine tablet comprises the following steps:
[0037] Step 1: passing clozapine through a 100-mesh sieve for subsequent use;
[0038] Step 2: take by weighing clozapine, microcrystalline cellulose, lactose by the prescription amount in claim 1 and place in the wet mixing granulator together and mix evenly;
[0039] Step 3: Weigh an appropriate amount of povidone, add water to dissolve and prepare an aqueous solution with a concentration of 3% to 5%;
[0040] Step 4: Add an appropriate amount of starch binder to the mixed powder in the previous step to make a suitable sof...
Embodiment 3
[0044] The invention provides a clozapine tablet and a preparation method thereof. The main ingredients and mass parts of the clozapine tablet are: 25 parts of clozapine, 61.2 parts of microcrystalline cellulose, 3.8 parts of lactose, povidone Appropriate amount, 0.5 parts of magnesium stearate, 1.4 parts of talcum powder, 0.4 parts of silicon dioxide;
[0045] Wherein, the preparation method of described clozapine tablet comprises the following steps:
[0046] Step 1: passing clozapine through a 100-mesh sieve for subsequent use;
[0047] Step 2: take by weighing clozapine, microcrystalline cellulose, lactose by the prescription amount in claim 1 and place in the wet mixing granulator together and mix evenly;
[0048] Step 3: Weigh an appropriate amount of povidone, dissolve it in water and prepare an aqueous solution with a concentration greater than 5%;
[0049] Step 4: Add an appropriate amount of starch binder to the mixed powder in the previous step to make a suitable ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com