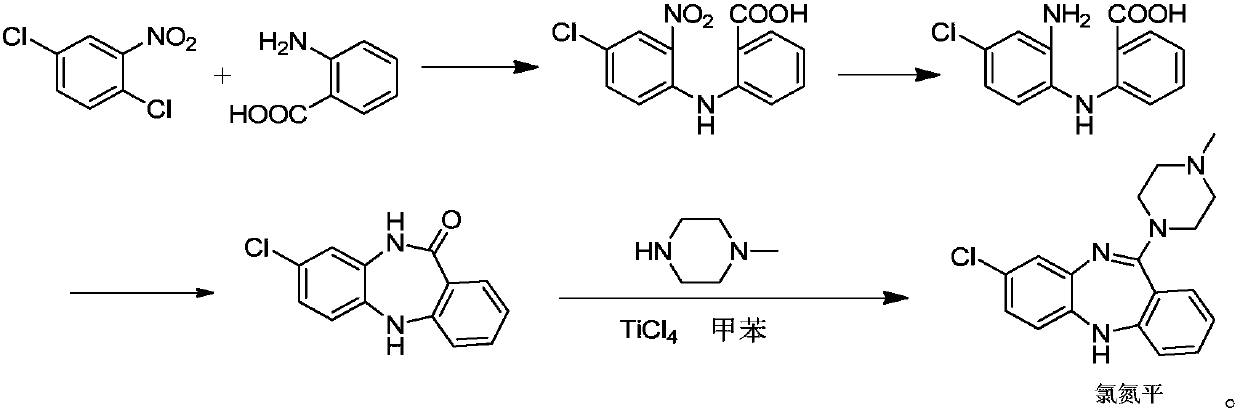
Synthetic method of key intermediate for preparing clozapine
A synthetic method, the technology of chlorophenyl, applied in the field of drug synthesis, can solve the problems of long reaction time, difficult industrial production, complex processing, etc., achieve good industrialization prospects, avoid cost increase, and avoid environmental pollution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
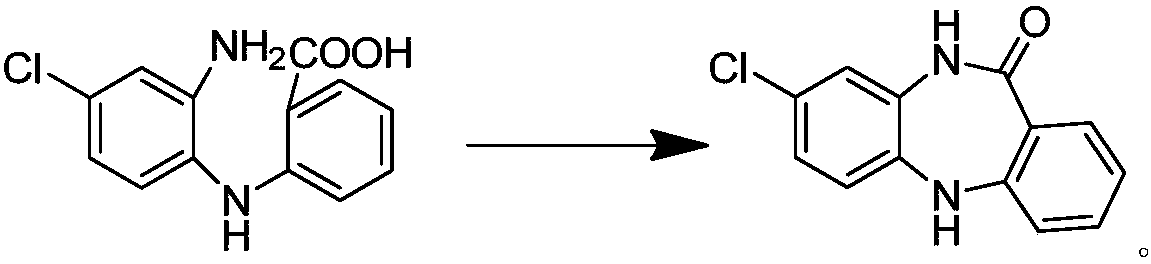
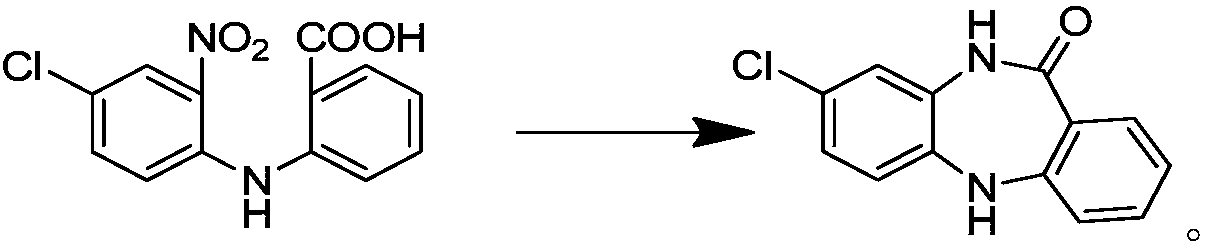
[0027] Add 15 g of 2-((2-amino-4-chlorophenyl) amino)benzoic acid into a three-necked reaction flask, add 100 g of sulfuric acid aqueous solution (mix 85 g of purified water with 15 g of sulfuric acid), and raise the temperature to reflux temperature (100-104 ℃), stirred for 4 hours. TLC followed the reaction. After the reaction was complete, the temperature was lowered to 58°C, filtered, washed with 3% sodium hydroxide solution until alkaline; washed with hot water at 58°C until nearly neutral, and dried; dried to obtain 8-chloro-5, 10-Dihydro-11H-dibenzo[b,e][1,4]-diazepine -11-one 12.5g, yield 89.5%, purity 99.111%.
Embodiment 2
[0029] 15 g of 2-((2-amino-4-chlorophenyl) amino) benzoic acid was added into a three-necked reaction flask, 100 g of hydrochloric acid aqueous solution (70 g of purified water mixed with 30 g of concentrated hydrochloric acid), and the temperature was raised to reflux temperature (100 -104°C), stirring for 4 hours. TLC followed the reaction. After the reaction was complete, the temperature was lowered to 58°C, filtered, washed with 3% sodium hydroxide solution until alkaline; washed with hot water at 58°C until nearly neutral, and dried; dried to obtain 8-chloro-5, 10-Dihydro-11H-dibenzo[b,e][1,4]-diazepine -11-one 11.8g, yield 84.5%, purity 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com