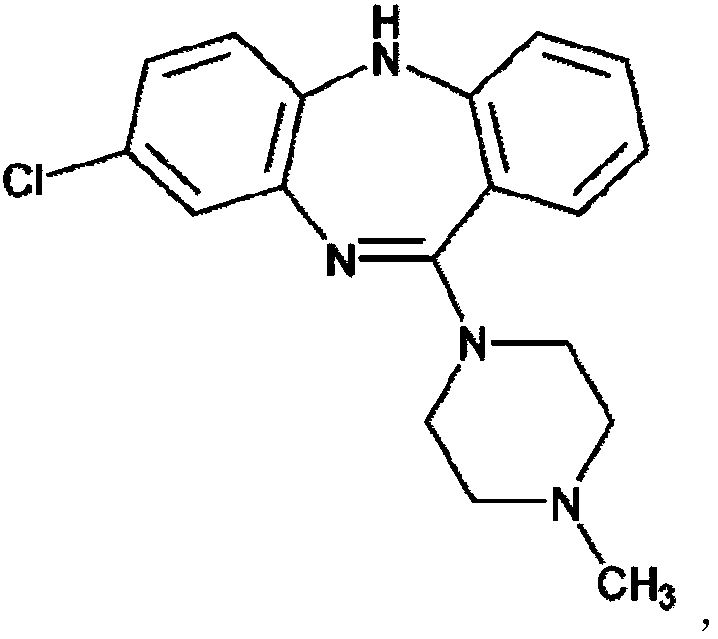
Clozapine tablet medicine composition and preparation method
A technology of clozapine tablets and compositions, applied in the field of tablet pharmaceutical compositions containing clozapine, capable of solving problems such as chemical stability defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
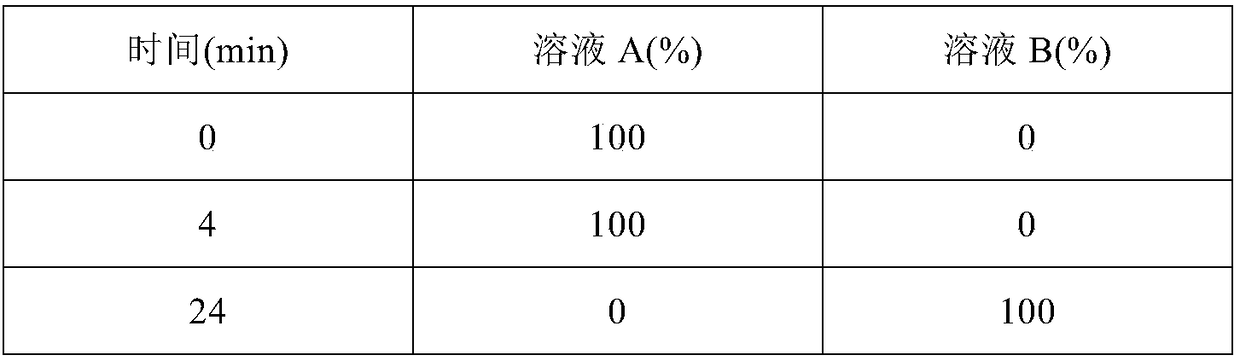
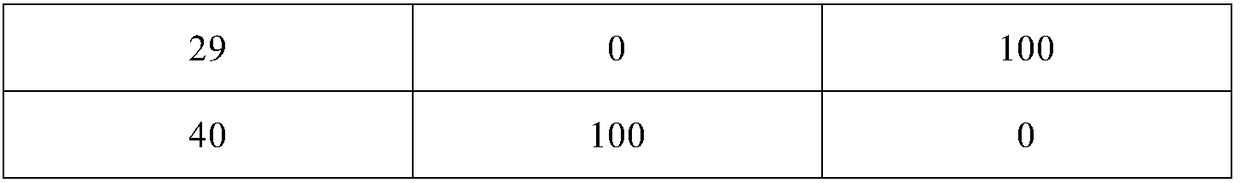
Image
Examples
Embodiment 1
[0135] Embodiment 1: prepare clozapine tablet
[0136] prescription:
[0137] Intragranular part
[0138] Preparation method: Mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 25% binder aqueous solution to make wet granules from this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
Embodiment 2
[0139] Embodiment 2: prepare clozapine tablet
[0140] prescription:
[0141] Intragranular part
[0142] Preparation method: mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 30% binder aqueous solution to prepare wet granules for this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
Embodiment 3
[0143] Embodiment 3: prepare clozapine tablet
[0144] prescription:
[0145] Intragranular part
[0146] Preparation method: mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 20% binder aqueous solution to prepare wet granules for this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com