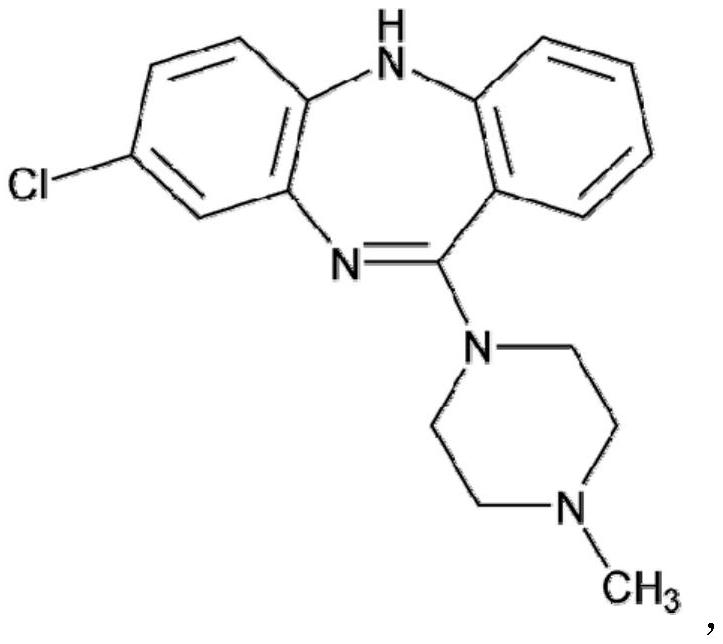
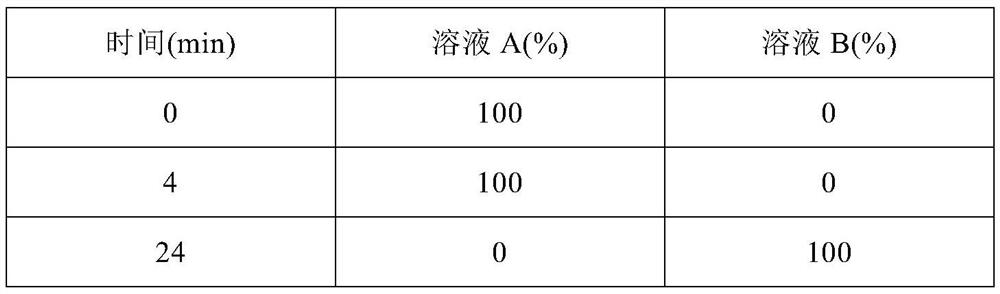
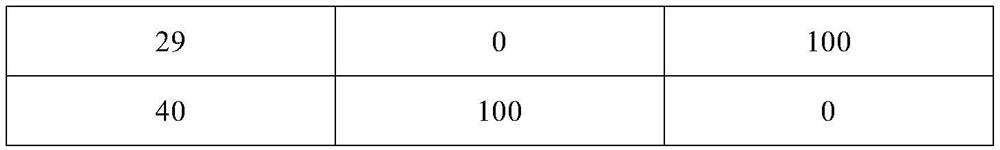
Clozapine tablet pharmaceutical composition and preparation method
A technology of clozapine tablets and compositions, applied in the field of tablet pharmaceutical compositions containing clozapine, can solve problems such as chemical stability defects, and achieve the effects of excellent tablet performance and excellent chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1: prepare clozapine tablet
[0131] prescription:
[0132] Intragranular part Clozapine 25mg lactose 48mg corn starch 7mg silica 0.95mg Polyvinylpyrrolidone K30 4.5mg extragranular part corn starch 7mg talcum powder 0.9mg Magnesium stearate 0.65mg
[0133] Preparation method: Mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 25% binder aqueous solution to make wet granules from this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
Embodiment 2
[0134] Embodiment 2: prepare clozapine tablet
[0135] prescription:
[0136] Intragranular part Clozapine 25mg lactose 20mg corn starch 6.7mg silica 1mg Polyvinylpyrrolidone K30 8mg extragranular part corn starch 13.3mg talcum powder 2mg Magnesium stearate 1mg
[0137] Preparation method: mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 30% binder aqueous solution to prepare wet granules for this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
Embodiment 3
[0138] Embodiment 3: prepare clozapine tablet
[0139] prescription:
[0140] Intragranular part Clozapine 25mg lactose 80mg corn starch 3mg silica 0.6mg Polyvinylpyrrolidone K30 2mg extragranular part corn starch 2mg talcum powder 0.4mg Magnesium stearate 0.2mg
[0141] Preparation method: mix clozapine, diluent, internally added part of disintegrant, and internally added part of glidant; use pre-prepared 20% binder aqueous solution to prepare wet granules for this mixture; dry the wet granules , 20-mesh sieve for granulation; add part of disintegrant, part of glidant, magnesium stearate, final blend to make all the materials evenly mixed; compress into tablets, to obtain. With the specification of 25mg / tablet, the inspection of relevant projects was carried out.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com