Sustained release system of clozapine solid liposome microparticle
A solid lipid, clozapine technology, applied in the directions of liposome delivery, nervous system diseases, medical preparations with inactive ingredients, etc. Speed, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
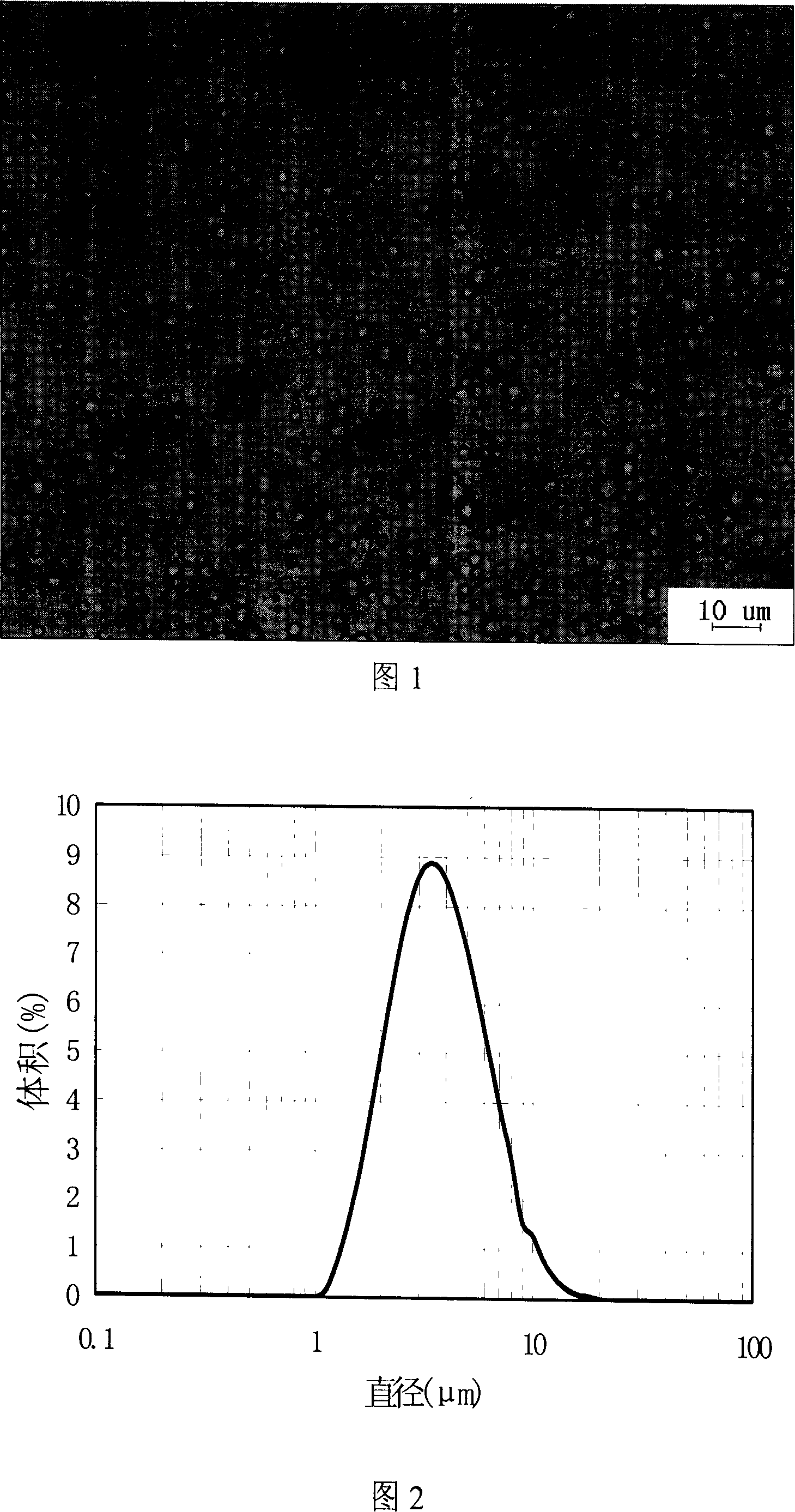
[0038]Under 85 ℃, the stearin of 5% (W / W, take emulsion as a benchmark, the same below) and 0.5% clozapine are mixed in dehydrated alcohol solvent, evaporate and recover solvent then; Disperse in an aqueous solution containing 2% lecithin / ox bile salt stabilizer at the same temperature under stirring; then process it with a high-shear dispersing emulsifier at 8000 rpm for about 15 minutes to form a highly dispersed O / W emulsion ; Finally, the emulsion is placed in a cold water bath at 5° C., and the fat phase is cooled and crystallized under mild magnetic stirring to obtain a suspension of clozapine solid lipid fine particles with an average particle size of 8 μm. Freeze-dry at a temperature of -50° C. and a pressure of 5.0 Pa for 24 hours to obtain dry microparticles.
Embodiment 2
[0040] 5% stearin and 0.5% clozapine were mixed in anhydrous ethanol solvent at 85°C, and then evaporated to recover the solvent; the molten mixture was dispersed at the same temperature containing 2% PVA (MW= 13000-23000) in the aqueous solution of the stabilizer; then treat it with a high-shear dispersing emulsifier at 10,000 rpm for about 15 minutes to form a highly dispersed O / W emulsion; finally place the emulsion in a cold water bath at 5°C, The lipid phase is cooled and crystallized under mild magnetic stirring to obtain a suspension of solid lipid microparticles with an average particle diameter of 5 μm encapsulating clozapine. Freeze-drying for 24 hours at a temperature of -50° C. and a pressure of 5.0 Pa to obtain dry microparticles.
Embodiment 3
[0042] 5% mixed esters (17% monoglyceride stearate, 54% diglyceride stearate, 29% triglyceride stearate) and 0.5% clozapine in absolute ethanol solvent at 85°C Mix and melt, then completely evaporate and reclaim the solvent; disperse the molten mixture in an aqueous solution containing 2% PVA (MW=13000-23000) stabilizer at the same temperature under magnetic stirring; then use 8000 rpm high shear Cut the dispersing emulsifier for about 15 minutes to form a highly dispersed O / W emulsion; finally place the emulsion in a cold water bath at 5°C, and cool and crystallize the lipid phase under gentle magnetic stirring to obtain the average particle size A suspension of clozapine solid lipid fine particles of 5 μm. Freeze-dry at a temperature of -50° C. and a pressure of 5.0 Pa for 24 hours to obtain dry microparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com